Human Mitragynine and 7-Hydroxymitragynine Pharmacokinetics after Single and Multiple Daily Doses of Oral Encapsulated Dried Kratom Leaf Powder
- PMID: 38474495
- PMCID: PMC10934259
- DOI: 10.3390/molecules29050984
Human Mitragynine and 7-Hydroxymitragynine Pharmacokinetics after Single and Multiple Daily Doses of Oral Encapsulated Dried Kratom Leaf Powder
Abstract
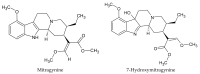
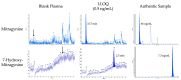
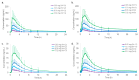
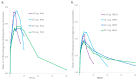
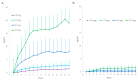
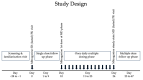
Kratom leaves, consumed by millions worldwide as tea or ground leaf powder, contain multiple alkaloids, with mitragynine being the most abundant and responsible for most effects. Mitragynine is a partial µ-opioid receptor agonist and competitive antagonist at κ- and δ-opioid receptors; however, unlike morphine, it does not activate the β-arrestin-2 respiratory depression pathway. Due to few human mitragynine data, the largest randomized, between-subject, double-blind, placebo-controlled, dose-escalation study of 500-4000 mg dried kratom leaf powder (6.65-53.2 mg mitragynine) was conducted. LC-MS/MS mitragynine and 7-hydroxymitragynine plasma concentrations were obtained after single and 15 daily doses. Mitragynine and 7-hydroxymitragynine Cmax increased dose proportionally, and AUC was slightly more than dose proportional. The median mitragynine Tmax was 1.0-1.3 h after single and 1.0-1.7 h after multiple doses; for 7-hydroxymitragynine Tmax, it was 1.2-1.8 h and 1.3-2.0 h. Steady-state mitragynine concentrations were reached in 8-9 days and 7-hydroxymitragynine within 7 days. The highest mean mitragynine T1/2 was 43.4 h after one and 67.9 h after multiple doses, and, for 7-hydroxymitragynine, it was 4.7 and 24.7 h. The mean 7-hydroxy-mitragynine/mitragynine concentration ratios were 0.20-0.31 after a single dose and decreased (0.15-0.21) after multiple doses. These mitragynine and 7-hydroxymitragynine data provide guidance for future clinical kratom dosing studies and an interpretation of clinical and forensic mitragynine and 7-hydroxymitragynine concentrations.
Keywords: 7-hydroxymitragynine; analytical toxicology; kratom; mass spectrometry; metabolism; mitragynine.
Conflict of interest statement
Johnson Foods, LLC., paid for this clinical study on its dried kratom leaf powder product MitraLeaf to be performed by SGS Nutrasource, Guelph, Ontario, Canada. Huestis, Brett, Bothmer and Atallah are paid consultants to Johnson Foods, LLC. Atallah is an employee of Della Terra Pharmaceuticals. Huestis and Atallah are also consultants for the American Kratom Association, which had no role in this study or publication.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

