Assessment of Hydrazone Derivatives for Enhanced Steel Corrosion Resistance in 15 wt.% HCl Environments: A Dual Experimental and Theoretical Perspective
- PMID: 38474497
- PMCID: PMC10935184
- DOI: 10.3390/molecules29050985
Assessment of Hydrazone Derivatives for Enhanced Steel Corrosion Resistance in 15 wt.% HCl Environments: A Dual Experimental and Theoretical Perspective
Abstract
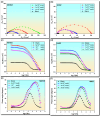
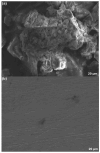
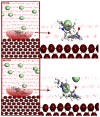
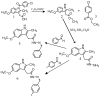
This study evaluates the corrosion inhibition capabilities of two novel hydrazone derivatives, (E)-2-(5-methoxy-2-methyl-1H-indol-3-yl)-N'-(4-methylbenzylidene)acetohydrazide (MeHDZ) and (E)-N'-benzylidene-2-(5-methoxy-2-methyl-1H-indol-3-yl)acetohydrazide (HHDZ), on carbon steel in a 15 wt.% HCl solution. A comprehensive suite of analytical techniques, including gravimetric analysis, potentiodynamic polarization (PDP), electrochemical impedance spectroscopy (EIS), and scanning electron microscopy (SEM), demonstrates their significant inhibition efficiency. At an optimal concentration of 5 × 10-3 mol/L, MeHDZ and HHDZ achieve remarkable inhibition efficiencies of 98% and 94%, respectively. EIS measurements reveal a dramatic reduction in effective double-layer capacitance (from 236.2 to 52.8 and 75.3 µF/cm2), strongly suggesting inhibitor adsorption on the steel surface. This effect is further corroborated by an increase in polarization resistance and a significant decrease in corrosion current density at optimal concentrations. Moreover, these inhibitors demonstrate sustained corrosion mitigation over extended exposure durations and maintain effectiveness even under elevated temperatures, highlighting their potential for diverse operational conditions. The adsorption process of these inhibitors aligns well with the Langmuir adsorption isotherm, implying physicochemical interactions at the carbon steel surface. Density functional tight-binding (DFTB) calculations and molecular dynamics simulations provide insights into the inhibitor-surface interaction mechanism, further elucidating the potential of these hydrazone derivatives as highly effective corrosion inhibitors in acidic environments.
Keywords: computational method; corrosion inhibition; corrosion testing; density functional tight-binding; hydrazone; pipeline; steel.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






Similar articles
-
Superior Long-Term Corrosion Inhibition of N80 Steel by New Eco-friendly Hydrazone-Based Compounds in a Simulated Oil Well Acidizing Environment: Establishing the Mechanism at the Molecular Level.Langmuir. 2022 Dec 27;38(51):15937-15949. doi: 10.1021/acs.langmuir.2c02132. Epub 2022 Dec 13. Langmuir. 2022. PMID: 36512730
-
Theoretical and Electrochemical Evaluation of Cannabis Sativa L. Extracts as Corrosion Inhibitors for Mild Steel in Acidic Medium.ChemistryOpen. 2025 May;14(5):e202400273. doi: 10.1002/open.202400273. Epub 2024 Dec 23. ChemistryOpen. 2025. PMID: 39715726 Free PMC article.
-
Unraveling the anti-corrosion mechanisms of a novel hydrazone derivative on steel in contaminated concrete pore solutions: An integrated study.J Adv Res. 2024 Apr;58:211-228. doi: 10.1016/j.jare.2023.08.016. Epub 2023 Aug 25. J Adv Res. 2024. PMID: 37634628 Free PMC article.
-
Innovation of Imine Metal Chelates as Corrosion Inhibitors at Different Media: A Collective Study.Int J Mol Sci. 2022 Aug 19;23(16):9360. doi: 10.3390/ijms23169360. Int J Mol Sci. 2022. PMID: 36012623 Free PMC article. Review.
-
Organic Compounds as Corrosion Inhibitors for Carbon Steel in HCl Solution: A Comprehensive Review.Materials (Basel). 2022 Mar 9;15(6):2023. doi: 10.3390/ma15062023. Materials (Basel). 2022. PMID: 35329474 Free PMC article. Review.
References
-
- Suvarapu L.N., Seo Y.K., Baek S.O., Ammireddy V.R. Review on Analytical and Biological Applications of Hydrazones and Their Metal Complexes. E-J. Chem. 2012;9:1288–1304. doi: 10.1155/2012/534617. - DOI
-
- Belskaya N.P., Dehaen W., Bakulev V.A. Synthesis and Properties of Hydrazones Bearing Amide, Thioamide and Amidine Functions. Arkivoc. 2010;2010:275–332. doi: 10.3998/ark.5550190.0011.108. - DOI
-
- Albayati M.R., Kansız S., Lgaz H., Kaya S., Dege N., Ali I.H., Salghi R., Chung I.-M. Synthesis, Experimental and Theoretical Characterization of (E)-2-((2,3-Dimethylphenyl)Amino)-N’-(Furan-2-Ylmethylene)Benzohydrazide. J. Mol. Struct. 2020;1219:128518. doi: 10.1016/j.molstruc.2020.128518. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

