Quercetin, a Flavonoid with Great Pharmacological Capacity
- PMID: 38474512
- PMCID: PMC10935205
- DOI: 10.3390/molecules29051000
Quercetin, a Flavonoid with Great Pharmacological Capacity
Abstract
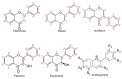
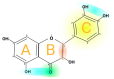
Quercetin is a flavonoid with a low molecular weight that belongs to the human diet's phenolic phytochemicals and nonenergy constituents. Quercetin has a potent antioxidant capacity, being able to capture reactive oxygen species (ROS), reactive nitrogen species (RNS), and reactive chlorine species (ROC), which act as reducing agents by chelating transition-metal ions. Its structure has five functional hydroxyl groups, which work as electron donors and are responsible for capturing free radicals. In addition to its antioxidant capacity, different pharmacological properties of quercetin have been described, such as carcinostatic properties; antiviral, antihypertensive, and anti-inflammatory properties; the ability to protect low-density lipoprotein (LDL) oxidation, and the ability to inhibit angiogenesis; these are developed in this review.
Keywords: antioxidant; free radicals; nanoparticles; pharmacological properties; quercetin.
Conflict of interest statement
Author Hector Fabián Nario-Chaidez owner of the company Sistemas y Servicios Oncológicos de Latinoamérica S.A. de C.V. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Flores G.P. Relaciones entre el Estrés Oxidativo y la Salud. Soc. Rural. Prod. Medio Ambiente. 2019;19:34.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

