Dearomatization of 3-Aminophenols for Synthesis of Spiro[chromane-3,1'-cyclohexane]-2',4'-dien-6'-ones via Hydride Transfer Strategy-Enabled [5+1] Annulations
- PMID: 38474524
- PMCID: PMC10933846
- DOI: 10.3390/molecules29051012
Dearomatization of 3-Aminophenols for Synthesis of Spiro[chromane-3,1'-cyclohexane]-2',4'-dien-6'-ones via Hydride Transfer Strategy-Enabled [5+1] Annulations
Abstract
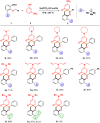
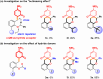
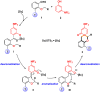
The Sc(OTf)3-catalyzed dearomative [5+1] annulations between readily available 3-aminophenols and O-alkyl ortho-oxybenzaldehydes were developed for synthesis of spiro[chromane-3,1'-cyclohexane]-2',4'-dien-6'-ones. The "two-birds-with-one-stone" strategy was disclosed by the dearomatization of phenols and direct α-C(sp3)-H bond functionalization of oxygen through cascade condensation/[1,5]-hydride transfer/dearomative-cyclization process. In addition, the antifungal activity assay and derivatizations of products were conducted to further enrich the utility of the structure.
Keywords: C(sp3)–H bond functionalization; antifungal activity; dearomatization; spiro[chromane-3,1′-cyclohexane]-2′,4′-dien-6′-ones.
Conflict of interest statement
Jia-Cheng Ge and Shuai-Shuai Li were employed by the company Hailir Pesticides and Chemicals Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Hydride Transfer-Enabled Dearomative Spirocyclization of Isoxazoles with O-Alkyl ortho-Oxybenzaldehydes.Org Lett. 2025 Feb 28;27(8):1835-1840. doi: 10.1021/acs.orglett.5c00001. Epub 2025 Feb 17. Org Lett. 2025. PMID: 39960009
-
Pd-Catalyzed Asymmetric Annulative Dearomatization of Phenols for Regio- and Enantioselective Synthesis of Spirocyclohexadienones.Org Lett. 2023 Aug 18;25(32):5963-5968. doi: 10.1021/acs.orglett.3c02051. Epub 2023 Aug 4. Org Lett. 2023. PMID: 37540111
-
Controllable Synthesis of N-Heterocycles via Hydride Transfer Strategy-Enabled Formal [5 + 1] and [5 + 2] Cyclizations.Org Lett. 2024 Jan 12;26(1):332-337. doi: 10.1021/acs.orglett.3c03986. Epub 2023 Dec 28. Org Lett. 2024. PMID: 38153999
-
The Cascade [1,5]-Hydride Shift/Intramolecular C(sp3)-H Activation: A Powerful Approach to the Construction of Spiro-Tetrahydroquinoline Skeleton.Front Chem. 2022 Apr 7;10:840934. doi: 10.3389/fchem.2022.840934. eCollection 2022. Front Chem. 2022. PMID: 35494642 Free PMC article. Review.
-
Palladium-catalyzed asymmetric dearomative cyclization in natural product synthesis.Org Biomol Chem. 2020 May 27. doi: 10.1039/d0ob00818d. Online ahead of print. Org Biomol Chem. 2020. PMID: 32459269 Review.
References
Grants and funding
- 21978144/National Natural Science Foundation of China
- 22208184/National Natural Science Foundation of China
- 2019KJM002/Support Plan on Science and Technology for Youth Innovation of Universities in Shandong Province
- 6651118009/Talents of High Level Scientific Research Foundation
- 6631115015/Talents of High Level Scientific Research Foundation
LinkOut - more resources
Full Text Sources

