Recent Trends in the Synthesis and Bioactivity of Coumarin, Coumarin-Chalcone, and Coumarin-Triazole Molecular Hybrids
- PMID: 38474540
- PMCID: PMC10934738
- DOI: 10.3390/molecules29051026
Recent Trends in the Synthesis and Bioactivity of Coumarin, Coumarin-Chalcone, and Coumarin-Triazole Molecular Hybrids
Abstract
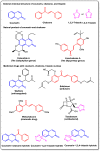
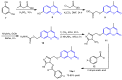
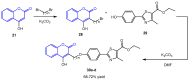
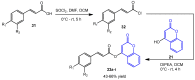
Molecular hybridization represents a new approach in drug discovery in which specific chromophores are strategically combined to create novel drugs with enhanced therapeutic effects. This innovative strategy leverages the strengths of individual chromophores to address complex biological challenges, synergize beneficial properties, optimize pharmacokinetics, and overcome limitations associated with single-agent therapies. Coumarins are documented to possess several bioactivities and have therefore been targeted for combination with other active moieties to create molecular hybrids. This review summarizes recent (2013-2023) trends in the synthesis of coumarins, as well as coumarin-chalcone and coumarin-triazole molecular hybrids. To cover the wide aspects of this area, we have included differently substituted coumarins, chalcones, 1,2,3- and 1,2,4-triazoles in this review and considered the point of fusion/attachment with coumarin to show the diversity of these hybrids. The reported syntheses mainly relied on well-established chemistry without the need for strict reaction conditions and usually produced high yields. Additionally, we discussed the bioactivities of the reported compounds, including antioxidative, antimicrobial, anticancer, antidiabetic, and anti-cholinesterase activities and commented on their IC50 where possible. Promising bioactivity results have been obtained so far. It is noted that mechanistic studies are infrequently found in the published work, which was also mentioned in this review to give the reader a better understanding. This review aims to provide valuable information to enable further developments in this field.
Keywords: biological activity; chalcone; coumarin; molecular hybridization; triazole.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







































Similar articles
-
Synthesis, biological evaluation and molecular docking of novel chalcone-coumarin hybrids as anticancer and antimalarial agents.Eur J Med Chem. 2014 Oct 6;85:65-76. doi: 10.1016/j.ejmech.2014.07.087. Epub 2014 Jul 24. Eur J Med Chem. 2014. PMID: 25078311
-
Design and synthesis of new coumarin hybrids and insight into their mode of antiproliferative action.Bioorg Med Chem. 2017 Feb 1;25(3):1066-1075. doi: 10.1016/j.bmc.2016.12.019. Epub 2016 Dec 19. Bioorg Med Chem. 2017. PMID: 28038941
-
Synthesis, in vitro anticancer and antibacterial activities and in silico studies of new 4-substituted 1,2,3-triazole-coumarin hybrids.Eur J Med Chem. 2016 Nov 29;124:794-808. doi: 10.1016/j.ejmech.2016.08.062. Epub 2016 Aug 31. Eur J Med Chem. 2016. PMID: 27639370
-
Recent Advances in Bioactive Flavonoid Hybrids Linked by 1,2,3-Triazole Ring Obtained by Click Chemistry.Molecules. 2021 Dec 30;27(1):230. doi: 10.3390/molecules27010230. Molecules. 2021. PMID: 35011463 Free PMC article. Review.
-
Coumarin Hybrids: Promising Scaffolds in the Treatment of Breast Cancer.Mini Rev Med Chem. 2019;19(17):1443-1458. doi: 10.2174/1389557519666190308122509. Mini Rev Med Chem. 2019. PMID: 30854961 Review.
Cited by
-
Step-by-step synthetic route to access eugenol-1,2,3-triazole-chalcone hybrid.MethodsX. 2024 Sep 12;13:102956. doi: 10.1016/j.mex.2024.102956. eCollection 2024 Dec. MethodsX. 2024. PMID: 39329152 Free PMC article.
-
Therapeutic potential of chalcone-1,2,3-triazole hybrids as anti-tumour agents: a systematic review and SAR studies.Future Med Chem. 2025 Feb;17(4):449-465. doi: 10.1080/17568919.2025.2458450. Epub 2025 Jan 31. Future Med Chem. 2025. PMID: 39886772
-
Mechanistic Aspects of [3+2] Cycloaddition Reaction of Trifluoroacetonitrile with Diarylnitrilimines in Light of Molecular Electron Density Theory Quantum Chemical Study.Molecules. 2024 Dec 29;30(1):85. doi: 10.3390/molecules30010085. Molecules. 2024. PMID: 39795142 Free PMC article.
-
Structural Features of Coumarin-1,2,4-Triazole Hybrids Important for Insecticidal Effects Against Drosophila melanogaster and Orius laevigatus (Fieber).Molecules. 2025 Apr 8;30(8):1662. doi: 10.3390/molecules30081662. Molecules. 2025. PMID: 40333563 Free PMC article.
-
Investigation of N-(2-oxo-2H-chromen-3-carbonyl)cytisine's Crystal Structure and Optical Properties.Materials (Basel). 2025 Jul 3;18(13):3153. doi: 10.3390/ma18133153. Materials (Basel). 2025. PMID: 40649642 Free PMC article.
References
-
- Fiorot R.G., Westphal R., Lemos B.C., Romagna R.A., Gonçalves P.R., Fernandes M.R.N., Ferreira C.V., Taranto A.G., Greco S.J. Synthesis, Molecular Modelling and Anticancer Activities of New Molecular Hybrids Containing 1,4-Naphthoquinone, 7-Chloroquinoline, 1,3,5-Triazine and Morpholine Cores as PI3K and AMPK Inhibitors in the Metastatic Melanoma Cells. J. Braz. Chem. Soc. 2019;30:1860–1873. doi: 10.21577/0103-5053.20190096. - DOI
-
- Burch J.D., Farand J., Colucci J., Sturino C., Ducharme Y., Friesen R.W., Lévesque J.-F., Gagné S., Wrona M., Therien A.G., et al. Naphthalene/quinoline amides and sulfonylureas as potent and selective antagonists of the EP4 receptor. Bioorg. Med. Chem. Lett. 2011;21:1041–1046. doi: 10.1016/j.bmcl.2010.12.014. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

