Novel Scaffold Agonists of the α2A Adrenergic Receptor Identified via Ensemble-Based Strategy
- PMID: 38474611
- PMCID: PMC10934406
- DOI: 10.3390/molecules29051097
Novel Scaffold Agonists of the α2A Adrenergic Receptor Identified via Ensemble-Based Strategy
Abstract
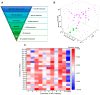
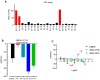
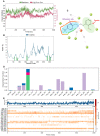
The α2A adrenergic receptor (α2A-AR) serves as a critical molecular target for sedatives and analgesics. However, α2A-AR ligands with an imidazole ring also interact with an imidazoline receptor as well as other proteins and lead to undesirable effects, motivating us to develop more novel scaffold α2A-AR ligands. For this purpose, we employed an ensemble-based ligand discovery strategy, integrating long-term molecular dynamics (MD) simulations and virtual screening, to identify new potential α2A-AR agonists with novel scaffold. Our results showed that compounds SY-15 and SY-17 exhibited significant biological effects in the preliminary evaluation of protein kinase A (PKA) redistribution assays. They also reduced levels of intracellular cyclic adenosine monophosphate (cAMP) in a dose-dependent manner. Upon treatment of the cells with 100 μM concentrations of SY-15 and SY-17, there was a respective decrease in the intracellular cAMP levels by 63.43% and 53.83%. Subsequent computational analysis was conducted to elucidate the binding interactions of SY-15 and SY-17 with the α2A-AR. The binding free energies of SY-15 and SY-17 calculated by MD simulations were -45.93 and -71.97 kcal/mol. MD simulations also revealed that both compounds act as bitopic agonists, occupying the orthosteric site and a novel exosite of the receptor simultaneously. Our findings of integrative computational and experimental approaches could offer the potential to enhance ligand affinity and selectivity through dual-site occupancy and provide a novel direction for the rational design of sedatives and analgesics.
Keywords: bitopic agonist; ensemble-based screening; molecular dynamics simulation; α2A-AR.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Schwinn D.A., Correa-Sales C., Page S.O., Maze M. Functional effects of activation of alpha-1 adrenoceptors by dexmedetomidine: In vivo and in vitro studies. J. Pharmacol. Exp. Ther. 1991;259:1147–1152. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

