Two-Phase Fermentation Systems for Microbial Production of Plant-Derived Terpenes
- PMID: 38474639
- PMCID: PMC10934027
- DOI: 10.3390/molecules29051127
Two-Phase Fermentation Systems for Microbial Production of Plant-Derived Terpenes
Abstract
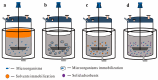
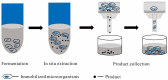
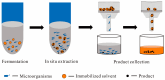
Microbial cell factories, renowned for their economic and environmental benefits, have emerged as a key trend in academic and industrial areas, particularly in the fermentation of natural compounds. Among these, plant-derived terpenes stand out as a significant class of bioactive natural products. The large-scale production of such terpenes, exemplified by artemisinic acid-a crucial precursor to artemisinin-is now feasible through microbial cell factories. In the fermentation of terpenes, two-phase fermentation technology has been widely applied due to its unique advantages. It facilitates in situ product extraction or adsorption, effectively mitigating the detrimental impact of product accumulation on microbial cells, thereby significantly bolstering the efficiency of microbial production of plant-derived terpenes. This paper reviews the latest developments in two-phase fermentation system applications, focusing on microbial fermentation of plant-derived terpenes. It also discusses the mechanisms influencing microbial biosynthesis of terpenes. Moreover, we introduce some new two-phase fermentation techniques, currently unexplored in terpene fermentation, with the aim of providing more thoughts and explorations on the future applications of two-phase fermentation technology. Lastly, we discuss several challenges in the industrial application of two-phase fermentation systems, especially in downstream processing.
Keywords: biosynthesis; downstream processing; in situ extraction; microbial cell factory; plant-derived terpenes; two-phase fermentation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Use of in situ solid-phase adsorption in microbial natural product fermentation development.J Ind Microbiol Biotechnol. 2013 May;40(5):411-25. doi: 10.1007/s10295-013-1247-9. Epub 2013 Mar 23. J Ind Microbiol Biotechnol. 2013. PMID: 23526181 Review.
-
Novel fermentation processes for manufacturing plant natural products.Curr Opin Biotechnol. 2014 Feb;25:17-23. doi: 10.1016/j.copbio.2013.08.009. Epub 2013 Sep 8. Curr Opin Biotechnol. 2014. PMID: 24484876 Review.
-
Engineering microbial cell factories for the production of plant natural products: from design principles to industrial-scale production.Microb Cell Fact. 2017 Jul 19;16(1):125. doi: 10.1186/s12934-017-0732-7. Microb Cell Fact. 2017. PMID: 28724386 Free PMC article. Review.
-
Progress in terpene synthesis strategies through engineering of Saccharomyces cerevisiae.Crit Rev Biotechnol. 2017 Dec;37(8):974-989. doi: 10.1080/07388551.2017.1299679. Epub 2017 Apr 20. Crit Rev Biotechnol. 2017. PMID: 28427280 Review.
-
[Synthetic biology of plants-derived medicinal natural products].Sheng Wu Gong Cheng Xue Bao. 2021 Jun 25;37(6):1931-1951. doi: 10.13345/j.cjb.210138. Sheng Wu Gong Cheng Xue Bao. 2021. PMID: 34227286 Review. Chinese.
Cited by
-
Microbial conversion of vegetable waste for flavor additives via solid-state fermentation: a comprehensive review.Front Nutr. 2025 Jun 24;12:1445189. doi: 10.3389/fnut.2025.1445189. eCollection 2025. Front Nutr. 2025. PMID: 40630173 Free PMC article. Review.
-
Ashbya gossypii as a versatile platform to produce sabinene from agro-industrial wastes.Fungal Biol Biotechnol. 2024 Oct 29;11(1):16. doi: 10.1186/s40694-024-00186-1. Fungal Biol Biotechnol. 2024. PMID: 39472989 Free PMC article.
-
Engineering terpene synthases and their substrates for the biocatalytic production of terpene natural products and analogues.Chem Commun (Camb). 2025 Feb 4;61(12):2468-2483. doi: 10.1039/d4cc05785f. Chem Commun (Camb). 2025. PMID: 39784321 Free PMC article. Review.
-
Compartmentalized Sesquiterpenoid Biosynthesis and Functionalization in the Chlamydomonas reinhardtii Plastid.Chembiochem. 2025 Feb 3;26(5):e202400902. doi: 10.1002/cbic.202400902. Epub 2024 Dec 5. Chembiochem. 2025. PMID: 39589357 Free PMC article.
References
-
- Cox-Georgian D., Ramadoss N., Dona C., Basu C. Therapeutic and Medicinal Uses of Terpenes. In: Joshee N., Dhekney S.A., Parajuli P., editors. Medicinal Plants: From Farm to Pharmacy. Springer International Publishing; Cham, Switzerland: 2019. pp. 333–359.
-
- Brahmkshatriya P.P., Brahmkshatriya P.S. Terpenes: Chemistry, Biological Role, and Therapeutic Applications. In: Ramawat K.G., Mérillon J.-M., editors. Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes. Springer; Berlin/Heidelberg, Germany: 2013. pp. 2665–2691.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

