Simultaneous Quantification of Nine Target Compounds in Traditional Korean Medicine, Bopyeo-Tang, Using High-Performance Liquid Chromatography-Photodiode Array Detector and Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry
- PMID: 38474683
- PMCID: PMC10934735
- DOI: 10.3390/molecules29051171
Simultaneous Quantification of Nine Target Compounds in Traditional Korean Medicine, Bopyeo-Tang, Using High-Performance Liquid Chromatography-Photodiode Array Detector and Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry
Abstract
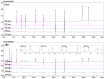
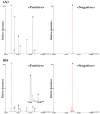
Bopyeo-tang (BPT) is composed of six medicinal herbs (Morus alba L., Rehmannia glutinosa (Gaertn.) DC., Panax ginseng C.A.Mey., Aster tataricus L.f., Astragalus propinquus Schischkin, and Schisandra chinensis (Turcz.) Baill.) and has been used for the treatment of lung diseases. This study focused on establishing an analytical method that can simultaneously quantify nine target compounds (i.e., hydroxymethylfurfural, mulberroside A, chlorogenic acid, calycosin-7-O-glucoside, 3,5-dicaffeoylquinic acid, quercetin, kaempferol, schizandrin, and gomisin A) from a BPT sample using high-performance liquid chromatography with a photodiode array detector (HPLC-PDA) and ultra-performance liquid chromatography with tandem mass spectrometry (UPLC-MS/MS). The separation of compounds in both analyses was performed on a C18 reversed-phase column using the gradient elution of water-acetonitrile as the mobile phase. In particular, the multiple reaction monitoring mode was applied for quick and accurate detection in UPLC-MS/MS analysis. As a result of analyzing the two methods, HPLC-PDA and UPLC-MS/MS, the coefficient of determination of the regression equation for each compound was ≥0.9952, and recovery was 85.99-106.40% (relative standard deviation (RSD) < 9.58%). Precision testing of the nine compounds was verified (RSD < 10.0%). The application of these analytical assays under optimized conditions for quantitative analysis of the BPT sample gave 0.01-4.70 mg/g. Therefore, these two assays could be used successfully to gather basic data for clinical research and the quality control of BPT.
Keywords: Bopyeo-tang; HPLC–PDA; UPLC–MS/MS; simultaneous quantification; traditional Korean medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
An Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometric Method for the Simultaneous Determination of Eighteen Marker Compounds in the Traditional Herbal Formula Bopyeo-Tang.Pharmaceuticals (Basel). 2024 Mar 8;17(3):352. doi: 10.3390/ph17030352. Pharmaceuticals (Basel). 2024. PMID: 38543138 Free PMC article.
-
Simultaneous Determination of Fourteen Marker Compounds in the Traditional Herbal Prescription, Geumgwesingihwan, Using Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry.Molecules. 2022 Jun 17;27(12):3890. doi: 10.3390/molecules27123890. Molecules. 2022. PMID: 35745012 Free PMC article.
-
Analysis of alkaloids in Coptis chinensis Franch by accelerated solvent extraction combined with ultra performance liquid chromatographic analysis with photodiode array and tandem mass spectrometry detections.Anal Chim Acta. 2008 Apr 21;613(2):184-95. doi: 10.1016/j.aca.2008.02.060. Epub 2008 Mar 5. Anal Chim Acta. 2008. PMID: 18395058
-
Forced degradation and impurity profiling: recent trends in analytical perspectives.J Pharm Biomed Anal. 2013 Dec;86:11-35. doi: 10.1016/j.jpba.2013.07.013. Epub 2013 Jul 31. J Pharm Biomed Anal. 2013. PMID: 23969330 Review.
-
Progress in the analysis of phytocannabinoids by HPLC and UPLC (or UHPLC) during 2020-2023.Phytochem Anal. 2024 Jul;35(5):927-989. doi: 10.1002/pca.3374. Epub 2024 Jun 4. Phytochem Anal. 2024. PMID: 38837522 Review.
Cited by
-
Development and Validation of an HPLC-PDA Method for Quality Control of Jwagwieum, an Herbal Medicine Prescription: Simultaneous Analysis of Nine Marker Compounds.Pharmaceuticals (Basel). 2025 Mar 27;18(4):481. doi: 10.3390/ph18040481. Pharmaceuticals (Basel). 2025. PMID: 40283917 Free PMC article.
-
Simultaneous Analysis of Thirteen Compounds in Yeokwisan Using High-Performance Liquid Chromatography-Photodiode Array Detection and Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry and Their Antioxidant Effects.Pharmaceuticals (Basel). 2024 Jun 4;17(6):727. doi: 10.3390/ph17060727. Pharmaceuticals (Basel). 2024. PMID: 38931394 Free PMC article.
References
-
- Yang L., Liu W., Hu Z., Yang M., Li J., Fan X., Pan H. A systems pharmacology approach for identifying the multiple mechanisms of action of the Wei Pi Xiao decoction for the treatment of gastric precancerous lesions. Evid. Based Complement. Alternat. Med. 2019;2019:1562707. doi: 10.1155/2019/1562707. - DOI - PMC - PubMed
-
- Heo J. Donguibogan. Namsandang; Seoul, Korea: 2007. p. 470.
-
- Shen J., Zhu X., Chen Y., Li W., Liu H., Chu C., Zhang Y., Xu C., Tong P., Yu X., et al. Bufei decoction improves lung-qi deficiency syndrome of chronic obstructive pulmonary disease in rats by regulating the balance of Th17/Treg cells. Evid. Based Complement. Alternat. Med. 2022;2022:1459232. doi: 10.1155/2022/1459232. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

