Metabolic Dysfunction-Associated Steatotic Liver Disease in a Dish: Human Precision-Cut Liver Slices as a Platform for Drug Screening and Interventions
- PMID: 38474754
- PMCID: PMC10934612
- DOI: 10.3390/nu16050626
Metabolic Dysfunction-Associated Steatotic Liver Disease in a Dish: Human Precision-Cut Liver Slices as a Platform for Drug Screening and Interventions
Abstract
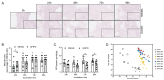
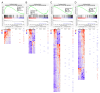
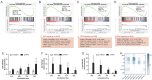
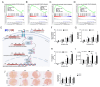
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a growing healthcare problem with limited therapeutic options. Progress in this field depends on the availability of reliable preclinical models. Human precision-cut liver slices (PCLSs) have been employed to replicate the initiation of MASLD, but a comprehensive investigation into MASLD progression is still missing. This study aimed to extend the current incubation time of human PCLSs to examine different stages in MASLD. Healthy human PCLSs were cultured for up to 96 h in a medium enriched with high sugar, high insulin, and high fatty acids to induce MASLD. PCLSs displayed hepatic steatosis, characterized by accumulated intracellular fat. The development of hepatic steatosis appeared to involve a time-dependent impact on lipid metabolism, with an initial increase in fatty acid uptake and storage, and a subsequent down-regulation of lipid oxidation and secretion. PCLSs also demonstrated liver inflammation, including increased pro-inflammatory gene expression and cytokine production. Additionally, liver fibrosis was also observed through the elevated production of pro-collagen 1a1 and tissue inhibitor of metalloproteinase-1 (TIMP1). RNA sequencing showed that the tumor necrosis factor alpha (TNFα) signaling pathway and transforming growth factor beta (TGFβ) signaling pathway were consistently activated, potentially contributing to the development of inflammation and fibrosis. In conclusion, the prolonged incubation of human PCLSs can establish a robust ex vivo model for MASLD, facilitating the identification and evaluation of potential therapeutic interventions.
Keywords: hepatic steatosis; liver fibrosis; long-term incubation; metabolic dysfunction-associated steatotic liver disease (MASLD); non-alcoholic fatty liver disease (NAFLD); precision-cut liver slices (PCLSs).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Willebrords J., Pereira I.V.A., Maes M., Yanguas S.C., Colle I., Bossche B.V.D., Silva T.C.D., Oliveira C.P.M.S.d., Andraus W., Alves V.A., et al. Strategies, models and biomarkers in experimental non-alcoholic fatty liver disease research. Prog. Lipid Res. 2015;59:106–125. doi: 10.1016/j.plipres.2015.05.002. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

