microRNA Expression Profile in Obesity-Induced Kidney Disease Driven by High-Fat Diet in Mice
- PMID: 38474819
- PMCID: PMC10934936
- DOI: 10.3390/nu16050691
microRNA Expression Profile in Obesity-Induced Kidney Disease Driven by High-Fat Diet in Mice
Abstract
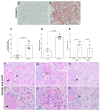
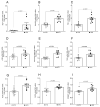
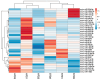
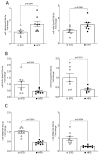
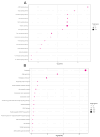
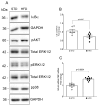
Obesity is one of the main causes of chronic kidney disease; however, the precise molecular mechanisms leading to the onset of kidney injury and dysfunction in obesity-associated nephropathy remain unclear. The present study aimed to unveil the kidney microRNA (miRNA) expression profile in a model of obesity-induced kidney disease in C57BL/6J mice using next-generation sequencing (NGS) analysis. High-fat diet (HFD)-induced obesity led to notable structural alterations in tubular and glomerular regions of the kidney, increased renal expression of proinflammatory and profibrotic genes, as well as an elevated renal expression of genes involved in cellular lipid metabolism. The miRNA sequencing analysis identified a set of nine miRNAs differentially expressed in the kidney upon HFD feeding, with miR-5099, miR-551b-3p, miR-223-3p, miR-146a-3p and miR-21a-3p showing the most significant differential expression between standard diet (STD) and HFD mice. A validation analysis showed that the expression levels of miR-5099, miR-551b-3p and miR-146a-3p were consistent with NGS results, while Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analyses revealed that these three validated miRNAs modulated target genes involved in metabolic and adipocytokine pathways, fatty acid and lipid metabolism, and inflammatory, senescence and profibrotic pathways. Our results suggest that differentially expressed miRNAs play pivotal roles in the intricate pathophysiology of obesity-associated kidney disease and could potentially create novel treatment strategies to counteract the deleterious effects of obesity on kidney function.
Keywords: chronic kidney disease; high-fat diet; kidney; lipotoxicity; miRNA; miRNA-seq; next-generation sequencing; obesity; obesity-induced kidney disease.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Di Angelantonio E., Bhupathiraju S.N., Wormser D., Gao P., Kaptoge S., de Gonzalez A.B., Cairns B.J., Huxley R., Jackson C.L., Joshy G., et al. Body-mass index and all-cause mortality: Individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. doi: 10.1016/S0140-6736(16)30175-1. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

