On-Chip Photonic Detection Techniques for Non-Invasive In Situ Characterizations at the Microfluidic Scale
- PMID: 38475065
- PMCID: PMC10933925
- DOI: 10.3390/s24051529
On-Chip Photonic Detection Techniques for Non-Invasive In Situ Characterizations at the Microfluidic Scale
Abstract
Microfluidics has emerged as a robust technology for diverse applications, ranging from bio-medical diagnostics to chemical analysis. Among the different characterization techniques that can be used to analyze samples at the microfluidic scale, the coupling of photonic detection techniques and on-chip configurations is particularly advantageous due to its non-invasive nature, which permits sensitive, real-time, high throughput, and rapid analyses, taking advantage of the microfluidic special environments and reduced sample volumes. Putting a special emphasis on integrated detection schemes, this review article explores the most relevant advances in the on-chip implementation of UV-vis, near-infrared, terahertz, and X-ray-based techniques for different characterizations, ranging from punctual spectroscopic or scattering-based measurements to different types of mapping/imaging. The principles of the techniques and their interest are discussed through their application to different systems.
Keywords: analytical chemistry; lab-on-a-chip; microfluidics; photonic detection; sensors; spectrometry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






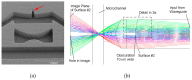
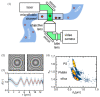
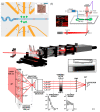
References
-
- Manz A., Graber N., Widmer H.M. Miniaturized Total Chemical Analysis Systems: A Novel Concept for Chemical Sensing. Sens. Actuators B Chem. 1990;1:244–248. doi: 10.1016/0925-4005(90)80209-I. - DOI
-
- Gravesen P., Branebjerg J., Jensen O.S. Microfluidics—A Review. J. Micromech. Microeng. 1993;3:168. doi: 10.1088/0960-1317/3/4/002. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

