Mechanochemical Encapsulation of Caffeine in UiO-66 and UiO-66-NH2 to Obtain Polymeric Composites by Extrusion with Recycled Polyamide 6 or Polylactic Acid Biopolymer
- PMID: 38475320
- PMCID: PMC10934872
- DOI: 10.3390/polym16050637
Mechanochemical Encapsulation of Caffeine in UiO-66 and UiO-66-NH2 to Obtain Polymeric Composites by Extrusion with Recycled Polyamide 6 or Polylactic Acid Biopolymer
Abstract
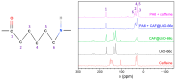
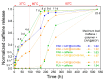
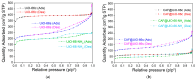
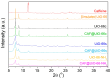
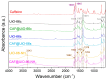
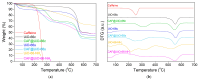
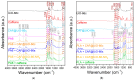
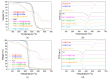
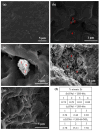
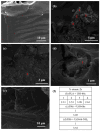
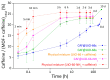
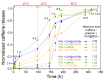
The development of capsules with additives that can be added to polymers during extrusion processing can lead to advances in the manufacturing of textile fabrics with improved and durable properties. In this work, caffeine (CAF), which has anti-cellulite properties, has been encapsulated by liquid-assisted milling in zirconium-based metal-organic frameworks (MOFs) with different textural properties and chemical functionalization: commercial UiO-66, UiO-66 synthesized without solvents, and UiO-66-NH2 synthesized in ethanol. The CAF@MOF capsules obtained through the grinding procedure have been added during the extrusion process to recycled polyamide 6 (PA6) and to a biopolymer based on polylactic acid (PLA) to obtain a load of approximately 2.5 wt% of caffeine. The materials have been characterized by various techniques (XRD, NMR, TGA, FTIR, nitrogen sorption, UV-vis, SEM, and TEM) that confirm the caffeine encapsulation, the preservation of caffeine during the extrusion process, and the good contact between the polymer and the MOF. Studies of the capsules and PA6 polymer+capsules composites have shown that release is slower when caffeine is encapsulated than when it is free, and the textural properties of UiO-66 influence the release more prominently than the NH2 group. However, an interaction is established between the biopolymer PLA and caffeine that delays the release of the additive.
Keywords: UiO-66; UiO-66-NH2; caffeine; metal organic framework; microencapsulation; polyamide; polylactic; textile composite.
Conflict of interest statement
The authors declare that this research work has been carried out within the LMP53_21 project that was funded by the Government of Aragon in collaboration with the company Nurel S.A. Elena Piera and Miguel Ángel Caballero were employed by the company Nurel. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









References
-
- Peng X., Umer M., Pervez M.N., Hasan K.M.F., Habib M.A., Islam M.S., Lin L., Xiong X., Naddeo V., Cai Y. Biopolymers-based microencapsulation technology for sustainable textiles development: A short review. Case Stud. Chem. Environ. Eng. 2023;7:100349. doi: 10.1016/j.cscee.2023.100349. - DOI
-
- García A., Ramos M., Sanahuja A., Garrigós M. Recent Trends in Microencapsulation for Smart and Active Innovative Textile Products. Curr. Org. Chem. 2018;22:1237–1248.
-
- Paseta L., Simón-Gaudó E., Gracia-Gorría F., Coronas J. Encapsulation of essential oils in porous silica and MOFs for trichloroisocyanuric acid tablets used for water treatment in swimming pools. Chem. Eng. J. 2016;292:28–34. doi: 10.1016/j.cej.2016.02.001. - DOI
-
- Shi M., Lu B., Li X., Jin Y., Ge M. Thermochromic luminescent fiber based on yellow thermochromic microcapsules: Preparation, properties, and potential application areas. Cellulose. 2021;28:5005–5018. doi: 10.1007/s10570-021-03858-y. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

