COF-Based Photocatalysts for Enhanced Synthesis of Hydrogen Peroxide
- PMID: 38475342
- PMCID: PMC10933836
- DOI: 10.3390/polym16050659
COF-Based Photocatalysts for Enhanced Synthesis of Hydrogen Peroxide
Abstract
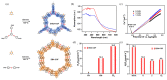
Covalent Organic Frameworks (COFs), with their intrinsic structural regularity and modifiable chemical functionality, have burgeoned as a pivotal material in the realm of photocatalytic hydrogen peroxide (H2O2) synthesis. This article reviews the recent advancements and multifaceted approaches employed in using the unique properties of COFs for high-efficient photocatalytic H2O2 production. We first introduced COFs and their advantages in the photocatalytic synthesis of H2O2. Subsequently, we spotlight the principles and evaluation of photocatalytic H2O2 generation, followed by various strategies for the incorporation of active sites aiming to optimize the separation and transfer of photoinduced charge carriers. Finally, we explore the challenges and future prospects, emphasizing the necessity for a deeper mechanistic understanding and the development of scalable and economically viable COF-based photocatalysts for sustainable H2O2 production.
Keywords: H2O2 photosynthesis; covalent organic framework; photosynthesis; solar conversion.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Zhang X., Zhang J., Miao J., Wen X., Chen C., Zhou B., Long M. Keto-enamine-based covalent organic framework with controllable anthraquinone moieties for superior H2O2 photosynthesis from O2 and water. Chem. Eng. J. 2023;466:143085. doi: 10.1016/j.cej.2023.143085. - DOI
-
- Wu M., Shan Z., Wang J., Liu T., Zhang G. Three-dimensional covalent organic framework with tty topology for enhanced photocatalytic hydrogen peroxide production. Chem. Eng. J. 2023;454:140121. doi: 10.1016/j.cej.2022.140121. - DOI
-
- Li J., Gao S.Y., Liu J., Ye S., Feng Y., Si D.H., Cao R. Use in Photoredox Catalysis of Stable Donor–Acceptor Covalent Organic Frameworks and Membrane Strategy. Adv. Funct. Mater. 2023;33:2305735. doi: 10.1002/adfm.202305735. - DOI
Publication types
LinkOut - more resources
Full Text Sources

