Cryostructuring of Polymeric Systems: 67 Properties and Microstructure of Poly(Vinyl Alcohol) Cryogels Formed in the Presence of Phenol or Bis-Phenols Introduced into the Aqueous Polymeric Solutions Prior to Their Freeze-Thaw Processing
- PMID: 38475358
- PMCID: PMC10935388
- DOI: 10.3390/polym16050675
Cryostructuring of Polymeric Systems: 67 Properties and Microstructure of Poly(Vinyl Alcohol) Cryogels Formed in the Presence of Phenol or Bis-Phenols Introduced into the Aqueous Polymeric Solutions Prior to Their Freeze-Thaw Processing
Abstract
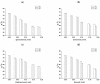
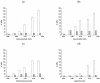
Poly(vinyl alcohol) (PVA) physical cryogels that contained the additives of o-, m-, and p-bis-phenols or phenol were prepared, and their physico-chemical characteristics and macroporous morphology and the solute release dynamics were evaluated. These phenolic additives caused changes in the viscosity of initial PVA solutions before their freeze-thaw processing and facilitated the growth in the rigidity of the resultant cryogels, while their heat endurance decreased. The magnitude of the effects depended on the interposition of phenolic hydroxyls in the molecules of the used additives and was stipulated by their H-bonding with PVA OH-groups. Subsequent rinsing of such "primary" cryogels with pure water led to the lowering of their rigidity. The average size of macropores inside these heterophase gels also depended on the additive type. It was found also that the release of phenolic substances from the additive-containing cryogels occurred via virtually a free diffusion mechanism; therefore, drug delivery systems such as PVA cryogels loaded with either pyrocatechol, resorcinol, hydroquinone, or phenol, upon the in vitro agar diffusion tests, exhibited antibacterial activity typical of these phenols. The promising biomedical potential of the studied nanocomposite gel materials is supposed.
Keywords: antimicrobial activity; drug release; phenols; physico-chemical properties; poly(vinyl alcohol) cryogel.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







References
-
- Kukharchik M.M., Baramboim N.K. Variation of the properties of poly(vinyl alcohol) aqueous solutions under cryogenic influence. Vysokomol. Soedin. 1972;14:843–846. (In Russian)
-
- Inoue T. Gelled Vinyl Alcohol Polymers and Articles Therefrom. 3,875,303. US Patent. 1975 April 1;
-
- Peppas N.A. Turbidimetric studies of aqueous poly(vinyl alcohol) solutions. Makromol. Chem. 1975;176:3433–3440. doi: 10.1002/macp.1975.021761125. - DOI
-
- Lozinsky V.I. Cryotropic gelation of poly(vinyl alcohol) Russ. Chem. Rev. 1998;67:573–586. doi: 10.1070/RC1998v067n07ABEH000399. - DOI
-
- Hassan C.M., Peppas N.A. Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv. Polym. Sci. 2000;153:37–65. doi: 10.1007/3-540-46414-X_2. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

