Differentially Expressed Genes Related to Isoflavone Biosynthesis in a Soybean Mutant Revealed by a Comparative Transcriptomic Analysis
- PMID: 38475431
- PMCID: PMC10934856
- DOI: 10.3390/plants13050584
Differentially Expressed Genes Related to Isoflavone Biosynthesis in a Soybean Mutant Revealed by a Comparative Transcriptomic Analysis
Abstract
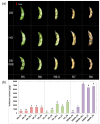
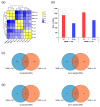
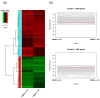
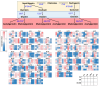
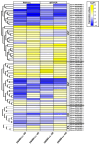
Soybean [Glycine max (L.) Merr.] isoflavones, which are secondary metabolites with various functions, are included in food, cosmetics, and medicine. However, the molecular mechanisms regulating the glycosylation and malonylation of isoflavone glycoconjugates remain unclear. In this study, we conducted an RNA-seq analysis to compare soybean genotypes with different isoflavone contents, including Danbaek and Hwanggeum (low-isoflavone cultivars) as well as DB-088 (high-isoflavone mutant). The transcriptome analysis yielded over 278 million clean reads, representing 39,156 transcripts. The analysis of differentially expressed genes (DEGs) detected 2654 up-regulated and 1805 down-regulated genes between the low- and high-isoflavone genotypes. The putative functions of these 4459 DEGs were annotated on the basis of GO and KEGG pathway enrichment analyses. These DEGs were further analyzed to compare the expression patterns of the genes involved in the biosynthesis of secondary metabolites and the genes encoding transcription factors. The examination of the relative expression levels of 70 isoflavone biosynthetic genes revealed the HID, IFS, UGT, and MAT expression levels were significantly up/down-regulated depending on the genotype and seed developmental stage. These expression patterns were confirmed by quantitative real-time PCR. Moreover, a gene co-expression analysis detected potential protein-protein interactions, suggestive of common functions. The study findings provide valuable insights into the structural genes responsible for isoflavone biosynthesis and accumulation in soybean seeds.
Keywords: Kyoto Encyclopedia of Genes and Genomes; RNA-seq; differentially expressed genes; gene ontology; isoflavone; soybean.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Dixit A., Antony J., Sharma N.K., Tiwari R.K. 12. Soybean constituents and their functional benefits. Res. Signpost. 2011;37:2.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

