Rosmarinic Acid Present in Lepechinia floribunda and Lepechinia meyenii as a Potent Inhibitor of the Adenylyl Cyclase gNC1 from Giardia lamblia
- PMID: 38475493
- PMCID: PMC10935199
- DOI: 10.3390/plants13050646
Rosmarinic Acid Present in Lepechinia floribunda and Lepechinia meyenii as a Potent Inhibitor of the Adenylyl Cyclase gNC1 from Giardia lamblia
Abstract
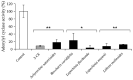
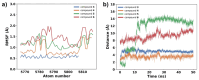
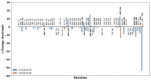
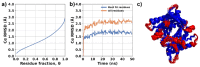
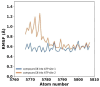
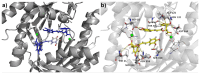
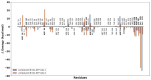
Giardiasis is a parasitosis caused by Giardia lamblia with significant epidemiological and clinical importance due to its high prevalence and pathogenicity. The lack of optimal therapies for treating this parasite makes the development of new effective chemical entities an urgent need. In the search for new inhibitors of the adenylyl cyclase gNC1 obtained from G. lamblia, 14 extracts from Argentinian native plants were screened. Lepechinia floribunda and L. meyenii extracts exhibited the highest gNC1 inhibitory activity, with IC50 values of 9 and 31 µg/mL, respectively. In silico studies showed rosmarinic acid, a hydroxycinnamic acid present in both mentioned species, to be a promising anti-gNC1 compound. This result was confirmed experimentally, with rosmarinic acid showing an IC50 value of 10.1 µM. Theoretical and experimental findings elucidate the molecular-level mechanism of rosmarinic acid, pinpointing the key interactions stabilizing the compound-enzyme complex and the binding site. These results strongly support that rosmarinic acid is a promising scaffold for developing novel compounds with inhibitory activity against gNC1, which could serve as potential therapeutic agents to treat giardiasis.
Keywords: Giardia lamblia; Lepechinia floribunda; Lepechinia meyenii; adenylyl cyclase inhibitors; molecular modeling; rosmarinic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
A Potent N-(piperidin-4-yl)-1H-pyrrole-2-carboxamide Inhibitor of Adenylyl Cyclase of G. lamblia: Biological Evaluation and Molecular Modelling Studies.ChemMedChem. 2021 Jul 6;16(13):2094-2105. doi: 10.1002/cmdc.202100037. Epub 2021 Apr 29. ChemMedChem. 2021. PMID: 33783977
-
Separation and Identification of Antioxidants and Aldose Reductase Inhibitors in Lepechinia meyenii (Walp.) Epling.Plants (Basel). 2021 Dec 15;10(12):2773. doi: 10.3390/plants10122773. Plants (Basel). 2021. PMID: 34961244 Free PMC article.
-
Synthesis and degradation of cAMP in Giardia lamblia: possible role and characterization of a nucleotidyl cyclase with a single cyclase homology domain.Biochem J. 2017 Nov 21;474(23):4001-4017. doi: 10.1042/BCJ20170590. Biochem J. 2017. PMID: 29054977
-
Helicobacter pylori Pathogenicity Islands and Giardia lamblia Cysteine Proteases in Role of Coinfection and Pathogenesis.Infect Drug Resist. 2022 Jan 6;15:21-34. doi: 10.2147/IDR.S346705. eCollection 2022. Infect Drug Resist. 2022. PMID: 35023934 Free PMC article. Review.
-
Mucosal Defense Against Giardia at the Intestinal Epithelial Cell Interface.Front Immunol. 2022 Feb 17;13:817468. doi: 10.3389/fimmu.2022.817468. eCollection 2022. Front Immunol. 2022. PMID: 35250996 Free PMC article. Review.
References
-
- Ryan U., Zahedi A. Advances in Parasitology. Volume 106. Elsevier; Amsterdam, The Netherlands: 2019. Molecular Epidemiology of Giardiasis from a Veterinary Perspective; pp. 209–254. - PubMed
Grants and funding
- PICT 2015-1769/Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación
- PICT-2018-03259/Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación
- PICT 2017-1381/Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación
- PICT 2019-2019-00721/Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación
- PICT-2020-SERIES-01627/Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación
LinkOut - more resources
Full Text Sources

