Evaluation of Parameters Affecting Agrobacterium-Mediated Transient Gene Expression in Industrial Hemp (Cannabis sativa L.)
- PMID: 38475511
- PMCID: PMC10934005
- DOI: 10.3390/plants13050664
Evaluation of Parameters Affecting Agrobacterium-Mediated Transient Gene Expression in Industrial Hemp (Cannabis sativa L.)
Abstract
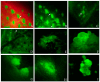
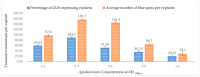
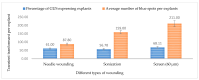
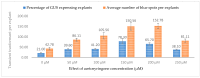
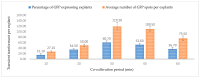
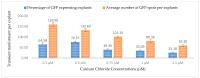
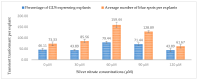
Industrial hemp Cannabis sativa L. is an economically important crop mostly grown for its fiber, oil, and seeds. Due to its increasing applications in the pharmaceutical industry and a lack of knowledge of gene functions in cannabinoid biosynthesis pathways, developing an efficient transformation platform for the genetic engineering of industrial hemp has become necessary to enable functional genomic and industrial application studies. A critical step in the development of Agrobacterium tumefaciens-mediated transformation in the hemp genus is the establishment of optimal conditions for T-DNA gene delivery into different explants from which whole plantlets can be regenerated. As a first step in the development of a successful Agrobacterium tumefaciens-mediated transformation method for hemp gene editing, the factors influencing the successful T-DNA integration and expression (as measured by transient β-glucuronidase (GUS) and Green Florescent Protein (GFP) expression) were investigated. In this study, the parameters for an agroinfiltration system in hemp, which applies to the stable transformation method, were optimized. In the present study, we tested different explants, such as 1- to 3-week-old leaves, cotyledons, hypocotyls, root segments, nodal parts, and 2- to 3-week-old leaf-derived calli. We observed that the 3-week-old leaves were the best explant for transient gene expression. Fully expanded 2- to 3-week-old leaf explants, in combination with 30 min of immersion time, 60 µM silver nitrate, 0.5 µM calcium chloride, 150 µM natural phenolic compound acetosyringone, and a bacterial density of OD600nm = 0.4 resulted in the highest GUS and GFP expression. The improved method of genetic transformation established in the present study will be useful for the introduction of foreign genes of interest, using the latest technologies such as genome editing, and studying gene functions that regulate secondary metabolites in hemp.
Keywords: Agrobacterium tumefaciens; Cannabis; GFP; GUS; genetic transformation; hemp; transient.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures









Similar articles
-
Improved Protocol for Efficient Agrobacterium-Mediated Transient Gene Expression in Medicago sativa L.Plants (Basel). 2024 Oct 26;13(21):2992. doi: 10.3390/plants13212992. Plants (Basel). 2024. PMID: 39519910 Free PMC article.
-
Establishment and optimization of a hemp (Cannabis sativa L.) agroinfiltration system for gene expression and silencing studies.Sci Rep. 2020 Feb 26;10(1):3504. doi: 10.1038/s41598-020-60323-9. Sci Rep. 2020. PMID: 32103049 Free PMC article.
-
Establishment of an Agrobacterium-mediated genetic transformation and CRISPR/Cas9-mediated targeted mutagenesis in Hemp (Cannabis Sativa L.).Plant Biotechnol J. 2021 Oct;19(10):1979-1987. doi: 10.1111/pbi.13611. Epub 2021 Jun 23. Plant Biotechnol J. 2021. PMID: 33960612 Free PMC article.
-
Metabolic Engineering Strategies of Industrial Hemp (Cannabis sativa L.): A Brief Review of the Advances and Challenges.Front Plant Sci. 2020 Dec 8;11:580621. doi: 10.3389/fpls.2020.580621. eCollection 2020. Front Plant Sci. 2020. PMID: 33363552 Free PMC article. Review.
-
Introduction to emerging industrial applications of cannabis (Cannabis sativa L.).Rend Lincei Sci Fis Nat. 2021;32(2):233-243. doi: 10.1007/s12210-021-00979-1. Epub 2021 Mar 19. Rend Lincei Sci Fis Nat. 2021. PMID: 33777341 Free PMC article. Review.
Cited by
-
A versatile, rapid Agrobacterium-mediated transient expression system for functional genomics studies in cannabis seedling.Planta. 2024 Jun 5;260(1):18. doi: 10.1007/s00425-024-04448-5. Planta. 2024. PMID: 38837044
-
Regeneration and Genetic Transformation in Eucalyptus Species, Current Research and Future Perspectives.Plants (Basel). 2024 Oct 11;13(20):2843. doi: 10.3390/plants13202843. Plants (Basel). 2024. PMID: 39458790 Free PMC article. Review.
-
CsCBDAS2-Driven Enhancement of Cannabinoid Biosynthetic Genes Using a High-Efficiency Transient Transformation System in Cannabis sativa 'Cheungsam'.Plants (Basel). 2025 May 14;14(10):1460. doi: 10.3390/plants14101460. Plants (Basel). 2025. PMID: 40431025 Free PMC article.
-
Potential Anti-Tumorigenic Properties of Diverse Medicinal Plants against the Majority of Common Types of Cancer.Pharmaceuticals (Basel). 2024 Apr 30;17(5):574. doi: 10.3390/ph17050574. Pharmaceuticals (Basel). 2024. PMID: 38794144 Free PMC article. Review.
-
Haploid Production in Cannabis sativa: Recent Updates, Prospects, and Perspectives.Biology (Basel). 2025 Jun 15;14(6):701. doi: 10.3390/biology14060701. Biology (Basel). 2025. PMID: 40563952 Free PMC article. Review.
References
-
- Deguchi M., Bogush D., Weeden H., Spuhler Z., Potlakayala S., Kondo T., Zhang Z.J., Rudrabhatla S. Establishment and optimization of hemp (Cannabis sativa L.) agroinfiltration system for gene expression and silencing studies. Sci. Rep. 2020;10:3504. doi: 10.1038/s41598-020-60323-9. - DOI - PMC - PubMed
-
- Gonçalves J., Rosado T., Soares S., Simão A.Y., Caramelo D., Luís Â., Fernández N., Barroso M., Gallardo E., Duarte A.P. Cannabis and its secondary metabolites: Their use as therapeutic drugs, toxicological aspects, and analytical determination. Medicines. 2019;6:31. doi: 10.3390/medicines6010031. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

