Plant Growth Promotion and Plant Disease Suppression Induced by Bacillus amyloliquefaciens Strain GD4a
- PMID: 38475518
- PMCID: PMC10934200
- DOI: 10.3390/plants13050672
Plant Growth Promotion and Plant Disease Suppression Induced by Bacillus amyloliquefaciens Strain GD4a
Abstract
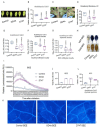
Botrytis cinerea, the causative agent of gray mold disease (GMD), invades plants to obtain nutrients and disseminates through airborne conidia in nature. Bacillus amyloliquefaciens strain GD4a, a beneficial bacterium isolated from switchgrass, shows great potential in managing GMD in plants. However, the precise mechanism by which GD4a confers benefits to plants remains elusive. In this study, an A. thaliana-B. cinerea-B. amyloliquefaciens multiple-scale interaction model was used to explore how beneficial bacteria play essential roles in plant growth promotion, plant pathogen suppression, and plant immunity boosting. Arabidopsis Col-0 wild-type plants served as the testing ground to assess GD4a's efficacy. Additionally, bacterial enzyme activity and targeted metabolite tests were conducted to validate GD4a's potential for enhancing plant growth and suppressing plant pathogens and diseases. GD4a was subjected to co-incubation with various bacterial, fungal, and oomycete pathogens to evaluate its antagonistic effectiveness in vitro. In vivo pathogen inoculation assays were also carried out to investigate GD4a's role in regulating host plant immunity. Bacterial extracellular exudate (BEE) was extracted, purified, and subjected to untargeted metabolomics analysis. Benzocaine (BEN) from the untargeted metabolomics analysis was selected for further study of its function and related mechanisms in enhancing plant immunity through plant mutant analysis and qRT-PCR analysis. Finally, a comprehensive model was formulated to summarize the potential benefits of applying GD4a in agricultural systems. Our study demonstrates the efficacy of GD4a, isolated from switchgrass, in enhancing plant growth, suppressing plant pathogens and diseases, and bolstering host plant immunity. Importantly, GD4a produces a functional bacterial extracellular exudate (BEE) that significantly disrupts the pathogenicity of B. cinerea by inhibiting fungal conidium germination and hypha formation. Additionally, our study identifies benzocaine (BEN) as a novel small molecule that triggers basal defense, ISR, and SAR responses in Arabidopsis plants. Bacillus amyloliquefaciens strain GD4a can effectively promote plant growth, suppress plant disease, and boost plant immunity through functional BEE production and diverse gene expression.
Keywords: Arabidopsis thaliana; Botrytis cinerea; bacterial extracellular exudates (BEE); benzocaine (BEN); metabolite analysis; systemic resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Plant Growth Promotion and Stress Tolerance Enhancement through Inoculation with Bacillus proteolyticus OSUB18.Biology (Basel). 2023 Dec 6;12(12):1495. doi: 10.3390/biology12121495. Biology (Basel). 2023. PMID: 38132321 Free PMC article.
-
Blue light regulates jasmonic acid synthesis via CRY1a and boosts antioxidant enzymes activity in Solanum lycopersicum to resist Botrytis cinerea.Plant Cell Rep. 2025 Jun 29;44(7):160. doi: 10.1007/s00299-025-03559-x. Plant Cell Rep. 2025. PMID: 40581909
-
Volatile Organic Compounds Emitted by the Biocontrol Agent Pythium oligandrum Contribute to Ginger Plant Growth and Disease Resistance.Microbiol Spectr. 2023 Aug 17;11(4):e0151023. doi: 10.1128/spectrum.01510-23. Epub 2023 Aug 3. Microbiol Spectr. 2023. PMID: 37534988 Free PMC article.
-
Immunogenicity and seroefficacy of pneumococcal conjugate vaccines: a systematic review and network meta-analysis.Health Technol Assess. 2024 Jul;28(34):1-109. doi: 10.3310/YWHA3079. Health Technol Assess. 2024. PMID: 39046101 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
Cited by
-
Biological Control of Root Rot of Strawberry by Bacillus amyloliquefaciens Strains CMS5 and CMR12.J Fungi (Basel). 2024 Jun 6;10(6):410. doi: 10.3390/jof10060410. J Fungi (Basel). 2024. PMID: 38921396 Free PMC article.
-
Bacillus amyloliquefaciens LM-1 Affects Multiple Cell Biological Processes in Magnaporthe oryzae to Suppress Rice Blast.Microorganisms. 2024 Jun 20;12(6):1246. doi: 10.3390/microorganisms12061246. Microorganisms. 2024. PMID: 38930628 Free PMC article.
-
Role of Methyl thiobutyrate to Botrytis cinerea on cucumber.Front Plant Sci. 2025 Apr 8;16:1551274. doi: 10.3389/fpls.2025.1551274. eCollection 2025. Front Plant Sci. 2025. PMID: 40265121 Free PMC article.
-
Isolation and Characterization of a Bacillus amyloliquefaciens Bacteriophage JBA6 and Its Endolysin PlyJBA6.J Microbiol Biotechnol. 2025 Apr 1;35:e2502026. doi: 10.4014/jmb.2502.02026. J Microbiol Biotechnol. 2025. PMID: 40174924 Free PMC article.
-
Exploring the potential of synthetic and biological fungicides for managing the fungus-farming ambrosia beetle Xylosandrus compactus.PLoS One. 2025 Jul 31;20(7):e0329063. doi: 10.1371/journal.pone.0329063. eCollection 2025. PLoS One. 2025. PMID: 40743054 Free PMC article.
References
-
- Wang S.-Y., Herrera-Balandrano D.D., Wang Y.-X., Shi X.-C., Chen X., Jin Y., Liu F.-Q., Laborda P. Biocontrol Ability of the Bacillus amyloliquefaciens Group, B. amyloliquefaciens, B. velezensis, B. nakamurai, and B. siamensis, for the Management of Fungal Postharvest Diseases: A Review. J. Agric. Food Chem. 2022;70:6591–6616. doi: 10.1021/acs.jafc.2c01745. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

