Serum ITIH5 as a novel diagnostic biomarker in cholangiocarcinoma
- PMID: 38475675
- PMCID: PMC11093185
- DOI: 10.1111/cas.16143
Serum ITIH5 as a novel diagnostic biomarker in cholangiocarcinoma
Abstract
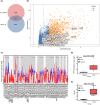
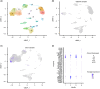
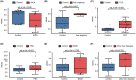
Cholangiocarcinoma often remains undetected until advanced stages due to the lack of reliable diagnostic markers. Our goal was to identify a unique secretory protein for cholangiocarcinoma diagnosis and differentiation from other malignancies, benign hepatobiliary diseases, and chronic liver conditions. We conducted bulk RNA-seq analysis to identify genes specifically upregulated in cholangiocarcinoma but not in most other cancers, benign hepatobiliary diseases, and chronic liver diseases focusing on exocrine protein-encoding genes. Single-cell RNA sequencing examined subcellular distribution. Immunohistochemistry and enzyme-linked immunosorbent assays assessed tissue and serum expression. Diagnostic performance was evaluated via receiver-operating characteristic (ROC) analysis. Inter-alpha-trypsin inhibitor heavy chain family member five (ITIH5), a gene encoding an extracellular protein, is notably upregulated in cholangiocarcinoma. This elevation is not observed in most other cancer types, benign hepatobiliary diseases, or chronic liver disorders. It is specifically expressed by malignant cholangiocytes. ITIH5 expression in cholangiocarcinoma tissues exceeded that in nontumorous bile duct, hepatocellular carcinoma, and nontumorous hepatic tissues. Serum ITIH5 levels were elevated in cholangiocarcinoma compared with controls (hepatocellular carcinoma, benign diseases, chronic hepatitis B, and healthy individuals). ITIH5 yielded areas under the ROC curve (AUCs) from 0.839 to 0.851 distinguishing cholangiocarcinoma from controls. Combining ITIH5 with carbohydrate antigen 19-9 (CA19-9) enhanced CA19-9's diagnostic effectiveness. In conclusion, serum ITIH5 may serve as a novel noninvasive cholangiocarcinoma diagnostic marker.
Keywords: ELISA; ITIH5; cholangiocarcinoma; diagnosis; serum marker.
© 2024 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Peptidase inhibitor 15 as a novel blood diagnostic marker for cholangiocarcinoma.EBioMedicine. 2019 Feb;40:422-431. doi: 10.1016/j.ebiom.2018.12.063. Epub 2019 Jan 9. EBioMedicine. 2019. PMID: 30638862 Free PMC article.
-
Application of Joint Detection of AFP, CA19-9, CA125 and CEA in Identification and Diagnosis of Cholangiocarcinoma.Asian Pac J Cancer Prev. 2015;16(8):3451-5. doi: 10.7314/apjcp.2015.16.8.3451. Asian Pac J Cancer Prev. 2015. PMID: 25921161
-
Detection of serum MMP-7 and MMP-9 in cholangiocarcinoma patients: evaluation of diagnostic accuracy.BMC Gastroenterol. 2009 Apr 30;9:30. doi: 10.1186/1471-230X-9-30. BMC Gastroenterol. 2009. PMID: 19405942 Free PMC article.
-
Circulating Biomarkers for Cholangiocarcinoma.Dig Dis. 2018;36(4):281-288. doi: 10.1159/000488342. Epub 2018 May 15. Dig Dis. 2018. PMID: 29807369 Review.
-
Cholangiocarcinoma: Present Status and Molecular Aspects of Diagnosis.Oncol Res. 2014;22(4):177-183. doi: 10.3727/096504015X14343704124386. Oncol Res. 2014. PMID: 26351206 Free PMC article. Review.
Cited by
-
High Expression of the Tumor Suppressor Protein ITIH5 in Cholangiocarcinomas Correlates with a Favorable Prognosis.Cancers (Basel). 2024 Oct 29;16(21):3647. doi: 10.3390/cancers16213647. Cancers (Basel). 2024. PMID: 39518085 Free PMC article.
-
The function of the inter-alpha-trypsin inhibitors in the development of disease.Front Med (Lausanne). 2024 Aug 1;11:1432224. doi: 10.3389/fmed.2024.1432224. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39149600 Free PMC article. Review.
References
-
- Sia D, Villanueva A, Friedman SL, Llovet JM. Liver cancer cell of origin, molecular class, and effects on patient prognosis. Gastroenterology. 2017;152:745‐761. - PubMed
-
- Charatcharoenwitthaya P, Enders FB, Halling KC, Lindor KD. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106‐1117. - PubMed
-
- Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657‐1669. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

