The enhanced antitumor activity of bispecific antibody targeting PD-1/PD-L1 signaling
- PMID: 38475778
- PMCID: PMC10935874
- DOI: 10.1186/s12964-024-01562-5
The enhanced antitumor activity of bispecific antibody targeting PD-1/PD-L1 signaling
Abstract
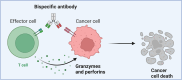
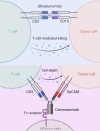
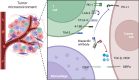
The programmed cell death 1 (PD-1) signaling pathway, a key player in immune checkpoint regulation, has become a focal point in cancer immunotherapy. In the context of cancer, upregulated PD-L1 on tumor cells can result in T cell exhaustion and immune evasion, fostering tumor progression. The advent of PD-1/PD-L1 inhibitor has demonstrated clinical success by unleashing T cells from exhaustion. Nevertheless, challenges such as resistance and adverse effects have spurred the exploration of innovative strategies, with bispecific antibodies (BsAbs) emerging as a promising frontier. BsAbs offer a multifaceted approach to cancer immunotherapy by simultaneously targeting PD-L1 and other immune regulatory molecules. We focus on recent advancements in PD-1/PD-L1 therapy with a particular emphasis on the development and potential of BsAbs, especially in the context of solid tumors. Various BsAb products targeting PD-1 signaling are discussed, highlighting their unique mechanisms of action and therapeutic potential. Noteworthy examples include anti-TGFβ × PD-L1, anti-CD47 × PD-L1, anti-VEGF × PD-L1, anti-4-1BB × PD-L1, anti-LAG-3 × PD-L1, and anti-PD-1 × CTLA-4 BsAbs. Besides, we summarize ongoing clinical studies evaluating the efficacy and safety of these innovative BsAb agents. By unraveling the intricacies of the tumor microenvironment and harnessing the synergistic effects of anti-PD-1/PD-L1 BsAbs, there exists the potential to elevate the precision and efficacy of cancer immunotherapy, ultimately enabling the development of personalized treatment strategies tailored to individual patient profiles.
Keywords: 4-1BB; Bispecific antibody; CD47; Cancer immunotherapy; PD-1; PD-L1; TGFβ; VEGF.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

