Transitions of blood immune endotypes and improved outcome by anakinra in COVID-19 pneumonia: an analysis of the SAVE-MORE randomized controlled trial
- PMID: 38475786
- PMCID: PMC10935809
- DOI: 10.1186/s13054-024-04852-z
Transitions of blood immune endotypes and improved outcome by anakinra in COVID-19 pneumonia: an analysis of the SAVE-MORE randomized controlled trial
Abstract
Background: Endotype classification may guide immunomodulatory management of patients with bacterial and viral sepsis. We aimed to identify immune endotypes and transitions associated with response to anakinra (human interleukin 1 receptor antagonist) in participants in the SAVE-MORE trial.
Methods: Adult patients hospitalized with radiological findings of PCR-confirmed severe pneumonia caused by SARS-CoV-2 and plasma-soluble urokinase plasminogen activator receptor levels of ≥ 6 ng/ml in the SAVE-MORE trial (NCT04680949) were characterized at baseline and days 4 and 7 of treatment using a previously defined 33-messenger RNA classifier to assign an immunological endotype in blood. Endpoints were changes in endotypes and progression to severe respiratory failure (SRF) associated with anakinra treatment.
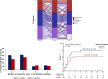
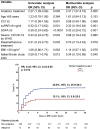
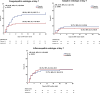
Results: At baseline, 23.2% of 393 patients were designated as inflammopathic, 41.1% as adaptive, and 35.7% as coagulopathic. Only 23.9% were designated as the same endotype at days 4 and 7 compared to baseline, while all other patients transitioned between endotypes. Anakinra-treated patients were more likely to remain in the adaptive endotype during 7-day treatment (24.4% vs. 9.9%; p < 0.001). Anakinra also protected patients with coagulopathic endotype at day 7 against SRF compared to placebo (27.8% vs. 55.9%; p = 0.013).
Conclusion: We identify an association between endotypes defined using blood transcriptome and anakinra therapy for COVID-19 pneumonia, with anakinra-treated patients shifting toward endotypes associated with a better outcome, mainly the adaptive endotype. Trial registration ClinicalTrials.gov, NCT04680949, December 23, 2020.
Keywords: Anakinra; COVID-19; Endotypes; Viral sepsis.
© 2024. The Author(s).
Conflict of interest statement
GND is an advisor or lecturer for Pfizer, Roche, Sanofi, Sobi, and Genesis and received research grants from Gilead and has served as PI in studies for Gilead, Novo Nordisk, Genkyotex, Regulus Therapeutics Inc, Tiziana Life Sciences, Pfizer, Amyndas Pharmaceuticals, CymaBay Therapeutics Inc., Sobi and Intercept Pharmaceuticals. MGN is a scientific founder of TTxD, Biotrip and Lemba and was supported by an ERC Advanced Grant (#833247) and a Spinoza grant of the Netherlands Organization for Scientific Research. PK is a co-founder of, holds stock options of and a consultant to Inflammatix, Inc. YH-B, UM, OL and TES are employees and stock option holders of Inflammatix, Inc. EJGΒ has received honoraria from Abbott Products Operations, bioMérieux, Brahms GmbH, GSK, InflaRx GmbH and Swedish Orphan BioVitrum; independent educational grants from Abbott Products Operations, bioMérieux Inc, InCyte, Johnson & Johnson, MSD, UCB and Swedish Orphan BioVitrum; and funding from the Horizon 2020 European Grants ImmunoSep and RISCinCOVID and the Horizon Health grant EPIC-CROWN-2 (granted to the Hellenic Institute for the Study of Sepsis). All other authors do not declare conflicts of interest.
Figures

References
-
- World Health Organization. Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019?gclid=Cj....
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

