Cryopreserved cGMP-compliant human pluripotent stem cell-derived hepatic progenitors rescue mice from acute liver failure through rapid paracrine effects on liver cells
- PMID: 38475825
- PMCID: PMC10935817
- DOI: 10.1186/s13287-024-03673-9
Cryopreserved cGMP-compliant human pluripotent stem cell-derived hepatic progenitors rescue mice from acute liver failure through rapid paracrine effects on liver cells
Abstract
Background: Liver transplantation remains the only curative treatment for end-stage liver diseases. Unfortunately, there is a drastic organ donor shortage. Hepatocyte transplantation emerged as a viable alternative to liver transplantation. Considering their unique expansion capabilities and their potency to be driven toward a chosen cell fate, pluripotent stem cells are extensively studied as an unlimited cell source of hepatocytes for cell therapy. It has been previously shown that freshly prepared hepatocyte-like cells can cure mice from acute and chronic liver failure and restore liver function.
Methods: Human PSC-derived immature hepatic progenitors (GStemHep) were generated using a new protocol with current good manufacturing practice compliant conditions from PSC amplification and hepatic differentiation to cell cryopreservation. The therapeutic potential of these cryopreserved cells was assessed in two clinically relevant models of acute liver failure, and the mode of action was studied by several analytical methods, including unbiased proteomic analyses.
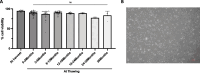
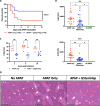
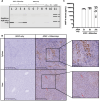
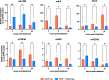
Results: GStemHep cells present an immature hepatic phenotype (alpha-fetoprotein positive, albumin negative), secrete hepatocyte growth factor and do not express major histocompatibility complex. A single dose of thawed GStemHep rescue mice from sudden death caused by acetaminophen and thioacetamide-induced acute liver failure, both in immunodeficient and immunocompetent animals in the absence of immunosuppression. Therapeutic biological effects were observed as soon as 3 h post-cell transplantation with a reduction in serum transaminases and in liver necrosis. The swiftness of the therapeutic effect suggests a paracrine mechanism of action of GStemHep leading to a rapid reduction of inflammation as well as a rapid cytoprotective effect with as a result a proteome reprograming of the host hepatocytes. The mode of action of GStemHep relie on the alleviation of inhibitory factors of liver regeneration, an increase in proliferation-promoting factors and a decrease in liver inflammation.
Conclusions: We generated cryopreserved and current good manufacturing practice-compliant human pluripotent stem cell-derived immature hepatic progenitors that were highly effective in treating acute liver failure through rapid paracrine effects reprogramming endogenous hepatocytes. This is also the first report highlighting that human allogeneic cells could be used as cryopreserved cells and in the absence of immunosuppression for human PSC-based regenerative medicine for acute liver failure.
Keywords: Acetaminophen; Acute liver failure; Cryopreservation; Hepatic progenitor cells; Pluripotent stem cells; Regenerative medicine; Thioacetamide; cGMP.
© 2024. The Author(s).
Conflict of interest statement
MG, AF, FD and THN are employees of Goliver Therapeutics. THN is a shareholder of Goliver Therapeutics. The remaining authors declare that the research was conducted in the absence of competing interests.
Figures


Similar articles
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
-
Interventions for paracetamol (acetaminophen) overdose.Cochrane Database Syst Rev. 2018 Feb 23;2(2):CD003328. doi: 10.1002/14651858.CD003328.pub3. Cochrane Database Syst Rev. 2018. PMID: 29473717 Free PMC article.
Cited by
-
Cell therapy for liver disorders: past, present and future.Nat Rev Gastroenterol Hepatol. 2025 May;22(5):329-342. doi: 10.1038/s41575-025-01050-2. Epub 2025 Mar 18. Nat Rev Gastroenterol Hepatol. 2025. PMID: 40102584 Review.
-
Human pluripotent stem cell-derived hepatic progenitors exhibit a partially hypoimmunogenic phenotype and actively inhibit immune responses.Front Immunol. 2025 Feb 25;16:1507317. doi: 10.3389/fimmu.2025.1507317. eCollection 2025. Front Immunol. 2025. PMID: 40070824 Free PMC article.
-
9-cis-Retinoic Acid Improves Disease Modelling in iPSC-Derived Liver Organoids.Cells. 2025 Jun 26;14(13):983. doi: 10.3390/cells14130983. Cells. 2025. PMID: 40643504 Free PMC article.
-
Bioartificial liver: Where lies the path ahead-A review.Hepatol Commun. 2025 Aug 15;9(9):e0788. doi: 10.1097/HC9.0000000000000788. eCollection 2025 Sep 1. Hepatol Commun. 2025. PMID: 40824256 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

