Application of fluorocarbon nanoparticles of 131I-fulvestrant as a targeted radiation drug for endocrine therapy on human breast cancer
- PMID: 38475902
- PMCID: PMC10929118
- DOI: 10.1186/s12951-024-02309-7
Application of fluorocarbon nanoparticles of 131I-fulvestrant as a targeted radiation drug for endocrine therapy on human breast cancer
Abstract
Background: Breast cancer is the most prevalent malignant tumor among women, with hormone receptor-positive cases constituting 70%. Fulvestrant, an antagonist for these receptors, is utilized for advanced metastatic hormone receptor-positive breast cancer. Yet, its inhibitory effect on tumor cells is not strong, and it lacks direct cytotoxicity. Consequently, there's a significant challenge in preventing recurrence and metastasis once cancer cells develop resistance to fulvestrant.
Method: To address these challenges, we engineered tumor-targeting nanoparticles termed 131I-fulvestrant-ALA-PFP-FA-NPs. This involved labeling fulvestrant with 131I to create 131I-fulvestrant. Subsequently, we incorporated the 131I-fulvestrant and 5-aminolevulinic acid (ALA) into fluorocarbon nanoparticles with folate as the targeting agent. This design facilitates a tri-modal therapeutic approach-endocrine therapy, radiotherapy, and PDT for estrogen receptor-positive breast cancer.
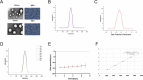
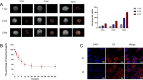
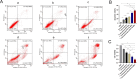
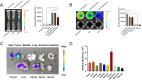
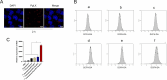
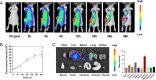
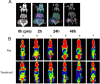
Results: Our in vivo and in vitro tests showed that the drug-laden nanoparticles effectively zeroed in on tumors. This targeting efficiency was corroborated using SPECT-CT imaging, confocal microscopy, and small animal fluorescence imaging. The 131I-fulvestrant-ALA-PFP-FA-NPs maintained stability and showcased potent antitumor capabilities due to the synergism of endocrine therapy, radiotherapy, and CR-PDT. Throughout the treatment duration, we detected no notable irregularities in hematological, biochemical, or histological evaluations.
Conclusion: We've pioneered a nanoparticle system loaded with radioactive isotope 131I, endocrine therapeutic agents, and a photosensitizer precursor. This system offers a combined modality of radiotherapy, endocrine treatment, and PDT for breast cancer.
Keywords: Breast cancer; Cerenkov radiation; Fulvestrant; Nanomedicine; Nuclear medicine; Photodynamic therapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interests.
Figures



Similar articles
-
Lasofoxifene as a potential treatment for therapy-resistant ER-positive metastatic breast cancer.Breast Cancer Res. 2021 May 12;23(1):54. doi: 10.1186/s13058-021-01431-w. Breast Cancer Res. 2021. PMID: 33980285 Free PMC article.
-
Minimizing adverse effects of Cerenkov radiation induced photodynamic therapy with transformable photosensitizer-loaded nanovesicles.J Nanobiotechnology. 2022 Apr 27;20(1):203. doi: 10.1186/s12951-022-01401-0. J Nanobiotechnology. 2022. PMID: 35477389 Free PMC article.
-
An evaluation of fulvestrant for the treatment of metastatic breast cancer.Expert Opin Pharmacother. 2019 Oct;20(15):1819-1829. doi: 10.1080/14656566.2019.1651293. Epub 2019 Sep 5. Expert Opin Pharmacother. 2019. PMID: 31486688 Review.
-
An evidence-based review of the outcome of fulvestrant plus a targeted agent versus fulvestrant alone in treating hormone receptor-positive endocrine therapy-resistant metastatic breast cancer.Arch Gynecol Obstet. 2019 Nov;300(5):1377-1382. doi: 10.1007/s00404-019-05304-8. Epub 2019 Oct 10. Arch Gynecol Obstet. 2019. PMID: 31599350 Review.
-
Synthesis and application of 131I-fulvestrant as a targeted radiation drug for endocrine therapy in human breast cancer.Oncol Rep. 2018 Mar;39(3):1215-1226. doi: 10.3892/or.2018.6212. Epub 2018 Jan 11. Oncol Rep. 2018. PMID: 29328488
References
-
- Masuda J, Sakai H, Tsurutani J, Tanabe Y, Masuda N, Iwasa T, et al. Efficacy, safety, and biomarker analysis of nivolumab in combination with abemaciclib plus endocrine therapy in patients with HR-positive HER2-negative metastatic breast cancer: a phase II study (WJOG11418B NEWFLAME trial) J Immunother Cancer. 2023;11(9):e007126. doi: 10.1136/jitc-2023-007126. - DOI - PMC - PubMed
-
- Overcoming Endocrine Resistance in Breast Cancer – PubMed. https://pubmed.ncbi.nlm.nih.gov/32289273/. Accessed 21 Sept 2023.
-
- Haddad TC, Suman VJ, D’Assoro AB, Carter JM, Giridhar KV, McMenomy BP, et al. Evaluation of alisertib alone or combined with fulvestrant in patients with endocrine-resistant advanced breast cancer: the phase 2 TBCRC041 randomized clinical trial. JAMA Oncol. 2023;9(6):815–824. doi: 10.1001/jamaoncol.2022.7949. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

