Reduction of monoclonal antibody viscosity using interpretable machine learning
- PMID: 38475982
- PMCID: PMC10939158
- DOI: 10.1080/19420862.2024.2303781
Reduction of monoclonal antibody viscosity using interpretable machine learning
Abstract
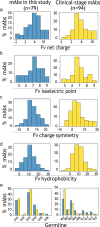
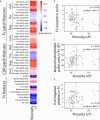
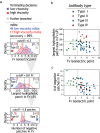
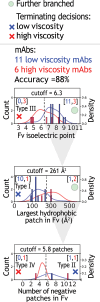
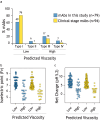
Early identification of antibody candidates with drug-like properties is essential for simplifying the development of safe and effective antibody therapeutics. For subcutaneous administration, it is important to identify candidates with low self-association to enable their formulation at high concentration while maintaining low viscosity, opalescence, and aggregation. Here, we report an interpretable machine learning model for predicting antibody (IgG1) variants with low viscosity using only the sequences of their variable (Fv) regions. Our model was trained on antibody viscosity data (>100 mg/mL mAb concentration) obtained at a common formulation pH (pH 5.2), and it identifies three key Fv features of antibodies linked to viscosity, namely their isoelectric points, hydrophobic patch sizes, and numbers of negatively charged patches. Of the three features, most predicted antibodies at risk for high viscosity, including antibodies with diverse antibody germlines in our study (79 mAbs) as well as clinical-stage IgG1s (94 mAbs), are those with low Fv isoelectric points (Fv pIs < 6.3). Our model identifies viscous antibodies with relatively high accuracy not only in our training and test sets, but also for previously reported data. Importantly, we show that the interpretable nature of the model enables the design of mutations that significantly reduce antibody viscosity, which we confirmed experimentally. We expect that this approach can be readily integrated into the drug development process to reduce the need for experimental viscosity screening and improve the identification of antibody candidates with drug-like properties.
Keywords: Antibody engineering; Fv; charge; computation; developability; formulation; hydrophobicity; in silico; isoelectric point; mutation.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


References
-
- Lee CH, Kang TH, Godon O, Watanabe M, Delidakis G, Gillis CM, Sterlin D, Hardy D, Cogné M, Macdonald LE, et al. Publisher correction: an engineered human Fc domain that behaves like a pH-toggle switch for ultra-long circulation persistence. Nat Commun. 2019;10(1):5031. doi: 10.1038/s41467-019-13108-2. - DOI - PMC - PubMed
-
- The Antibody Society . Therapeutic monoclonal antibodies approved or in regulatory review. [accessed 2024 Jan 5]. www.antibodysociety.org/antibody-therapeutics-product-data
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
