Transfusion-related errors and associated adverse reactions and blood product wastage as reported to the National Healthcare Safety Network Hemovigilance Module, 2014-2022
- PMID: 38476028
- PMCID: PMC11299550
- DOI: 10.1111/trf.17775
Transfusion-related errors and associated adverse reactions and blood product wastage as reported to the National Healthcare Safety Network Hemovigilance Module, 2014-2022
Abstract
Background: Transfusion-related errors are largely preventable but may lead to blood product wastage and adverse reactions, resulting in patient harm. In the United States, the incidence of transfusion-related errors is poorly understood nationally. We used data from the National Healthcare Safety Network (NHSN) Hemovigilance Module to describe and quantify transfusion-related errors, as well as associated transfusion-related adverse reactions and blood product wastage.
Methods: During 2014-2022, data from the NHSN Hemovigilance Module were used to analyze errors, including near misses (errors with no transfusion), incidents (errors with transfusion), and associated serious adverse reactions (severe, life-threatening, or death).
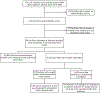
Results: During 2014-2022, 80 acute care facilities (75 adult; 5 pediatric) reported 63,900 errors. Most errors occurred during patient blood sample collection (21,761, 34.1%) and blood sample handling (16,277, 25.5%). Less than one-fifth of reported errors (9822, 15.4%) had a completed incident form. Of those, 8780 (89.3%) were near misses and 1042 (10.7%) incidents. More than a third of near misses (3363, 38.3%) were associated with a discarded blood product, resulting in 4862 discarded components. Overall, 87 adverse reactions were associated with errors; six (7%) were serious.
Conclusions: Over half of the transfusion-related errors reported to the Hemovigilance Module occurred during blood sample collection or sample handling. Some serious adverse reactions identified were associated with errors, suggesting that additional safety interventions may be beneficial. Increased participation in the Hemovigilance Module could enhance generalizability and further inform policy development regarding error prevention.
Keywords: biovigilance; blood wastage; hemovigilance; transfusion incidents; transfusion‐related errors.
Published 2024. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The authors have disclosed no conflicts of interest.
Figures

Similar articles
-
Transfusion-related adverse reactions: Data from the National Healthcare Safety Network Hemovigilance Module - United States, 2013-2018.Transfusion. 2021 May;61(5):1424-1434. doi: 10.1111/trf.16362. Epub 2021 Apr 20. Transfusion. 2021. PMID: 33880771
-
Transfusion-related adverse reactions reported to the National Healthcare Safety Network Hemovigilance Module, United States, 2010 to 2012.Transfusion. 2015 Apr;55(4):709-18. doi: 10.1111/trf.12918. Epub 2014 Nov 5. Transfusion. 2015. PMID: 25371300 Free PMC article.
-
How is national recipient hemovigilance conducted in the United States?Transfusion. 2015 Apr;55(4):703-7. doi: 10.1111/trf.12980. Epub 2015 Jan 7. Transfusion. 2015. PMID: 25565577 Free PMC article.
-
A Comparison of Transfusion-Related Adverse Reactions Among Apheresis Platelets, Whole Blood-Derived Platelets, and Platelets Subjected to Pathogen Reduction Technology as Reported to the National Healthcare Safety Network Hemovigilance Module.Transfus Med Rev. 2021 Apr;35(2):78-84. doi: 10.1016/j.tmrv.2021.03.003. Epub 2021 Apr 2. Transfus Med Rev. 2021. PMID: 33934903 Free PMC article. Review.
-
Adverse events of red blood cell transfusions in patients with sickle cell disease.Transfus Apher Sci. 2022 Oct;61(5):103557. doi: 10.1016/j.transci.2022.103557. Epub 2022 Aug 29. Transfus Apher Sci. 2022. PMID: 36064527 Free PMC article. Review.
Cited by
-
Blood Borders: State laws, vaccine misinformation, and the threat to the United States blood supply.Transfusion. 2025 Aug;65(8):1534-1542. doi: 10.1111/trf.18312. Epub 2025 Jun 16. Transfusion. 2025. PMID: 40521642 Free PMC article. No abstract available.
References
-
- Public Health Agency of Canada. Transfusion error surveillance system (TESS), 2012–2016 report. Canada: Centre for Communicable Diseases and Infection Control, Infectious Disease Prevention and Control Branch, Public Health Agency of Canada; 2021.
-
- BBC. Warrington Hospital staff sacked after patient given wrong blood type. Liverpool: British Broadcasting Corporation (BBC) News; 2014.
-
- Substantive Hearing 27 October and 15 December 2017. London: Nursing and Midwifery Council Fitness to Practise Committee; 2017.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

