LncRNA CTBP1-AS inhibits TP63-mediated activation of S100A14 during prostate cancer progression
- PMID: 38476086
- PMCID: PMC11093200
- DOI: 10.1111/cas.16138
LncRNA CTBP1-AS inhibits TP63-mediated activation of S100A14 during prostate cancer progression
Abstract
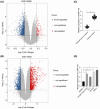
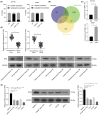
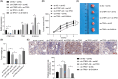
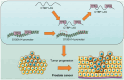
Long noncoding RNAs (lncRNAs) have emerged as important molecules and potential new targets for human cancers. This study investigates the function of lncRNA CTBP1 antisense RNA (CTBP1-AS) in prostate cancer (PCa) and explores the entailed molecular mechanism. Aberrantly expressed genes potentially correlated with PCa progression were probed using integrated bioinformatics analyses. A cohort of 68 patients with PCa was included, and their tumor and para-cancerous tissues were collected. CTBP1-AS was highly expressed in PCa tissues and cells and associated with poor patient prognosis. By contrast, tumor protein p63 (TP63) and S100 calcium binding protein A14 (S100A14) were poorly expressed in the PCa tissues and cells. CTBP1-AS did not affect TP63 expression; however it blocked the TP63-mediated transcriptional activation of S100A14, thereby reducing its expression. CTBP1-AS silencing suppressed proliferation, apoptosis resistance, migration, invasion, and tumorigenicity of PCa cell lines, while its overexpression led to inverse results. The malignant phenotype of cells was further weakened by TP63 overexpression but restored following artificial S100A14 silencing. In conclusion, this study demonstrates that CTBP1-AS plays an oncogenic role in PCa by blocking TP63-mediated transcriptional activation of S100A14. This may provide insight into the management of PCa.
Keywords: CTBP1‐AS; S100A14; TP63; prostate cancer; transcription.
© 2024 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

