Structural Determinants of Stimuli-Responsiveness in Amphiphilic Macromolecular Nano-assemblies
- PMID: 38476148
- PMCID: PMC10927256
- DOI: 10.1016/j.progpolymsci.2023.101765
Structural Determinants of Stimuli-Responsiveness in Amphiphilic Macromolecular Nano-assemblies
Abstract
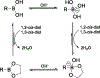
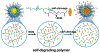
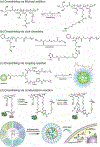
Stimuli-responsive nano-assemblies from amphiphilic macromolecules could undergo controlled structural transformations and generate diverse macroscopic phenomenon under stimuli. Due to the controllable responsiveness, they have been applied for broad material and biomedical applications, such as biologics delivery, sensing, imaging, and catalysis. Understanding the mechanisms of the assembly-disassembly processes and structural determinants behind the responsive properties is fundamentally important for designing the next generation of nano-assemblies with programmable responsiveness. In this review, we focus on structural determinants of assemblies from amphiphilic macromolecules and their macromolecular level alterations under stimuli, such as the disruption of hydrophilic-lipophilic balance (HLB), depolymerization, decrosslinking, and changes of molecular packing in assemblies, which eventually lead to a series of macroscopic phenomenon for practical purposes. Applications of stimuli-responsive nano-assemblies in delivery, sensing and imaging were also summarized based on their structural features. We expect this review could provide readers an overview of the structural considerations in the design and applications of nanoassemblies and incentivize more explorations in stimuli-responsive soft matters.
Keywords: Amphiphilic macromolecules; Biomedical applications; Disassembly; Nanoassemblies; Self-assembly; Stimuli-responsive; Structural determinants.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


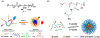





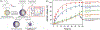







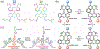

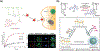



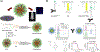
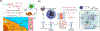

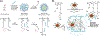
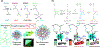


Similar articles
-
Supramolecular disassembly of facially amphiphilic dendrimer assemblies in response to physical, chemical, and biological stimuli.Acc Chem Res. 2014 Jul 15;47(7):2200-11. doi: 10.1021/ar500143u. Epub 2014 Jun 17. Acc Chem Res. 2014. PMID: 24937682 Free PMC article.
-
Multiple-stimuli-responsiveness and conformational inversion of smart supramolecular nanoparticles assembled from spin labeled amphiphilic random copolymers.J Colloid Interface Sci. 2021 Mar;585:237-249. doi: 10.1016/j.jcis.2020.11.042. Epub 2020 Nov 19. J Colloid Interface Sci. 2021. PMID: 33285462
-
Stimuli-responsive nano-assemblies for remotely controlled drug delivery.J Control Release. 2020 Jun 10;322:566-592. doi: 10.1016/j.jconrel.2020.03.051. Epub 2020 Apr 8. J Control Release. 2020. PMID: 32276006 Review.
-
Stimuli-Responsive Plasmonic Assemblies and Their Biomedical Applications.Nano Today. 2021 Feb;36:101014. doi: 10.1016/j.nantod.2020.101014. Epub 2020 Nov 8. Nano Today. 2021. PMID: 33250931 Free PMC article.
-
Stimuli-Responsive Block Copolymer-Based Assemblies for Cargo Delivery and Theranostic Applications.Polymers (Basel). 2016 Jul 22;8(7):268. doi: 10.3390/polym8070268. Polymers (Basel). 2016. PMID: 30974545 Free PMC article. Review.
Cited by
-
Strategies, Challenges, and Prospects of Nanoparticles in Gynecological Malignancies.ACS Omega. 2024 Aug 23;9(36):37459-37504. doi: 10.1021/acsomega.4c04573. eCollection 2024 Sep 10. ACS Omega. 2024. PMID: 39281920 Free PMC article. Review.
-
Predicting the Key Properties of a Modified Product to Pre-Select a Pluronic F127 Modification Scheme for Preparing High-Quality Nano-Micelles.Polymers (Basel). 2025 Jan 27;17(3):349. doi: 10.3390/polym17030349. Polymers (Basel). 2025. PMID: 39940552 Free PMC article.
-
Antibody-Directing Antibody Conjugates (ADACs) Enabled by Orthogonal Click Chemistry for Targeted Intracellular Delivery.Small. 2024 Nov;20(47):e2402874. doi: 10.1002/smll.202402874. Epub 2024 Aug 20. Small. 2024. PMID: 39162119
-
Polymersome-based nanomotors: preparation, motion control, and biomedical applications.Chem Sci. 2025 Apr 3;16(17):7106-7129. doi: 10.1039/d4sc08283d. eCollection 2025 Apr 30. Chem Sci. 2025. PMID: 40206551 Free PMC article. Review.
-
Molecular-Based Nanoplatform Leads to the Formation of a Self-Indicating Responsive Drug Delivery System.Molecules. 2025 Apr 16;30(8):1782. doi: 10.3390/molecules30081782. Molecules. 2025. PMID: 40333797 Free PMC article.
References
-
- Aubert S, Bezagu M, Spivey AC, Arseniyadis S. Spatial and temporal control of chemical processes. Nat Rev Chem 2019;3:706–22. 10.1038/s41570-019-0139-6. - DOI
-
- Lutz J-F, Lehn J-M, Meijer EW, Matyjaszewski K. From precision polymers to complex materials and systems. Nat Rev Mater 2016;1:16024. 10.1038/natrevmats.2016.24. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
