Yolk-shell smart polymer microgels and their hybrids: fundamentals and applications
- PMID: 38476178
- PMCID: PMC10929002
- DOI: 10.1039/d4ra00035h
Yolk-shell smart polymer microgels and their hybrids: fundamentals and applications
Abstract
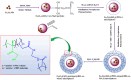
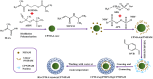
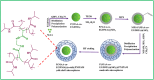
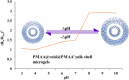
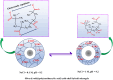
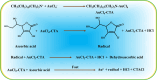
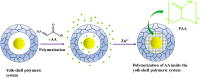
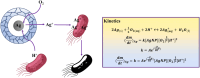
Yolk-shell microgels and their hybrids have attained great importance in modern-day research owing to their captivating features and potential uses. This manuscript provides the strategies for preparation, classification, properties and current applications of yolk-shell microgels and their hybrids. Some of the yolk-shell microgels and their hybrids are identified as smart polymer yolk-shell microgels and smart hybrid microgels, respectively, as they react to changes in particular environmental stimuli such as pH, temperature and ionic strength of the medium. This unique behavior makes them a perfect candidate for utilization in drug delivery, selective catalysis, adsorption of metal ions, nanoreactors and many other fields. This review demonstrates the contemporary progress along with suggestions and future perspectives for further research in this specific field.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
Authors declare no conflict of interest.
Figures

















Similar articles
-
Fundamentals and applications of acrylamide based microgels and their hybrids: a review.RSC Adv. 2019 May 7;9(24):13838-13854. doi: 10.1039/c9ra00699k. eCollection 2019 Apr 30. RSC Adv. 2019. PMID: 35519604 Free PMC article. Review.
-
UV-Vis spectroscopy in the characterization and applications of smart microgels and metal nanoparticle-decorated smart microgels: a critical review.RSC Adv. 2024 Nov 29;14(51):38120-38134. doi: 10.1039/d4ra07643e. eCollection 2024 Nov 25. RSC Adv. 2024. PMID: 39619811 Free PMC article. Review.
-
A Critical Review of Palladium Nanoparticles Decorated in Smart Microgels.Polymers (Basel). 2023 Aug 30;15(17):3600. doi: 10.3390/polym15173600. Polymers (Basel). 2023. PMID: 37688226 Free PMC article. Review.
-
Recent developments in chitosan based microgels and their hybrids.Int J Biol Macromol. 2024 Mar;260(Pt 1):129409. doi: 10.1016/j.ijbiomac.2024.129409. Epub 2024 Jan 13. Int J Biol Macromol. 2024. PMID: 38224801 Review.
-
Physicochemical aspects of inorganic nanoparticles stabilized in N-vinyl caprolactam based microgels for various applications.RSC Adv. 2021 Jan 4;11(2):978-995. doi: 10.1039/d0ra09327k. eCollection 2020 Dec 24. RSC Adv. 2021. PMID: 35423699 Free PMC article. Review.
Cited by
-
Nanoarchitectonics for structural tailoring of yolk-shell architectures for electrochemical applications.Sci Technol Adv Mater. 2024 Oct 29;25(1):2420664. doi: 10.1080/14686996.2024.2420664. eCollection 2024. Sci Technol Adv Mater. 2024. PMID: 39539602 Free PMC article. Review.
References
-
- Liu J. Qiao S. Z. Chen J. S. Lou X. W. D. Xing X. Lu G. Q. M. Chem. Commun. 2011;47:12578–12591. - PubMed
-
- Xing S. Tan L. H. Chen T. Yang Y. Chen H. Chem. Commun. 2009;13:1653–1654. - PubMed
-
- Liu J. Cheng J. Che R. Xu J. Liu M. Liu Z. ACS Appl. Mater. Interfaces. 2013;5:2503–2509. - PubMed
-
- Wang S. Zhang M. Zhang W. ACS Catal. 2011;1:207–211.
-
- Chen X. Song L. Li X. Zhang L. Li L. Zhang X. Wang C. Chem. Eng. J. 2020;389:124416.
Publication types
LinkOut - more resources
Full Text Sources

