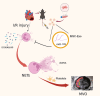
MSC-Derived Exosomes Mitigate Myocardial Ischemia/Reperfusion Injury by Reducing Neutrophil Infiltration and the Formation of Neutrophil Extracellular Traps
- PMID: 38476275
- PMCID: PMC10928923
- DOI: 10.2147/IJN.S436925
MSC-Derived Exosomes Mitigate Myocardial Ischemia/Reperfusion Injury by Reducing Neutrophil Infiltration and the Formation of Neutrophil Extracellular Traps
Abstract
Introduction: Acute inflammatory storm is a major cause of myocardial ischemia/reperfusion (I/R) injury, with no effective treatment currently available. The excessive aggregation of neutrophils is correlated with an unfavorable prognosis in acute myocardial infarction (AMI) patients. Exosomes derived from mesenchymal stromal cells (MSC-Exo) have certain immunomodulatory potential and might be a therapeutic application. Therefore, we investigated the protective role of MSC-Exo in modulating neutrophil infiltration and formation of neutrophil extracellular traps (NETs) following myocardial I/R injury.
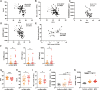
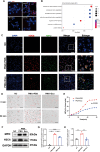
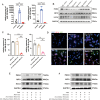
Methods: Exosomes were isolated from the supernatant of MSCs using a gradient centrifugation method. We used flow cytometry, histochemistry, and immunofluorescence to detect the changes of neutrophils post-intravenous MSC-Exo injection. Additionally, cardiac magnetic resonance (CMR) and thioflavin S experiments were applied to detect microvascular obstruction (MVO). The NLR family pyrin domain containing 3 (NLRP3) inflammasome was examined for mechanism exploration. Primary neutrophils were extracted for in vitro experiment. Antibody of Ly6G was given to depleting the neutrophils in mice for verification the effect of MSC-Exo. Finally, we analyzed the MiRNA sequence of MSC-Exo and verified it in vitro.
Results: MSC-Exo administration reduced neutrophil infiltration and NETs formation after myocardial I/R. MSC-Exo treatment also could attenuate the activation of NLRP3 inflammasome both in vivo and in vitro. At the same time, the infarction size and MVO following I/R injury were reduced by MSC-Exo. Moreover, systemic depletion of neutrophils partly negated the therapeutic effects of MSC-Exo. Up-regulation of miR-199 in neutrophils has been shown to decrease the expression of NETs formation after stimulation.
Discussion: Our results demonstrated that MSC-Exo mitigated myocardial I/R injury in mice by modulating neutrophil infiltration and NETs formation. This study provides novel insights into the potential therapeutic application of MSC-Exo for myocardial ischemia/reperfusion injury.
Keywords: exosomes; mesenchymal stromal cells; mir-199; myocardial ischemia/reperfusion injury; neutrophil extracellular traps.
© 2024 Feng et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization.Cardiovasc Res. 2019 Jun 1;115(7):1205-1216. doi: 10.1093/cvr/cvz040. Cardiovasc Res. 2019. PMID: 30753344 Free PMC article.
-
Cathelicidin aggravates myocardial ischemia/reperfusion injury via activating TLR4 signaling and P2X7R/NLRP3 inflammasome.J Mol Cell Cardiol. 2020 Feb;139:75-86. doi: 10.1016/j.yjmcc.2019.12.011. Epub 2020 Jan 23. J Mol Cell Cardiol. 2020. PMID: 31982429
-
Intravenous Administration of Mesenchymal Stem Cell-Derived Exosome Alleviates Spinal Cord Injury by Regulating Neutrophil Extracellular Trap Formation through Exosomal miR-125a-3p.Int J Mol Sci. 2024 Feb 18;25(4):2406. doi: 10.3390/ijms25042406. Int J Mol Sci. 2024. PMID: 38397083 Free PMC article.
-
Inflammasome Activation and Neutrophil Extracellular Traps in Atherosclerosis.J Atheroscler Thromb. 2025 May 1;32(5):535-549. doi: 10.5551/jat.RV22033. Epub 2025 Jan 18. J Atheroscler Thromb. 2025. PMID: 39828369 Free PMC article. Review.
-
Mesenchymal Stem Cell-Derived Exosomal Noncoding RNAs as Alternative Treatments for Myocardial Ischemia-Reperfusion Injury: Current Status and Future Perspectives.J Cardiovasc Transl Res. 2023 Oct;16(5):1085-1098. doi: 10.1007/s12265-023-10401-w. Epub 2023 Jun 7. J Cardiovasc Transl Res. 2023. PMID: 37286924 Free PMC article. Review.
Cited by
-
Inflammatory Cell-Targeted Delivery Systems for Myocardial Infarction Treatment.Bioengineering (Basel). 2025 Feb 19;12(2):205. doi: 10.3390/bioengineering12020205. Bioengineering (Basel). 2025. PMID: 40001724 Free PMC article. Review.
-
Advanced Nanomaterial Platforms for Targeted Therapy of Myocardial Ischemia-Reperfusion Injury.Research (Wash D C). 2025 Aug 5;8:0822. doi: 10.34133/research.0822. eCollection 2025. Research (Wash D C). 2025. PMID: 40765995 Free PMC article. Review.
-
Therapeutic Potential of Umbilical Cord MSC-Derived Exosomes in a Severe Dry Eye Rat Model: Enhancing Corneal Protection and Modulating Inflammation.Biomedicines. 2025 May 11;13(5):1174. doi: 10.3390/biomedicines13051174. Biomedicines. 2025. PMID: 40427001 Free PMC article.
-
Mesenchymal stem cell exosome therapy: current research status in the treatment of neurodegenerative diseases and the possibility of reversing normal brain aging.Stem Cell Res Ther. 2025 Feb 21;16(1):76. doi: 10.1186/s13287-025-04160-5. Stem Cell Res Ther. 2025. PMID: 39985030 Free PMC article. Review.
-
MSC-Derived Extracellular Vesicles: Roles and Molecular Mechanisms for Tissue Repair.Int J Nanomedicine. 2025 Jun 21;20:7953-7974. doi: 10.2147/IJN.S525394. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40568355 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

