Ultrarapid Iron Polymaltose Infusions Are Safe for Management of Iron Deficiency
- PMID: 38476307
- PMCID: PMC10928866
- DOI: 10.1159/000527794
Ultrarapid Iron Polymaltose Infusions Are Safe for Management of Iron Deficiency
Abstract
Introduction: Iron deficiency is a common condition, especially among patients with kidney and heart failure and inflammatory bowel disease. Intravenous iron is the preferred method of treatment in these patients, but it usually requires prolonged iron polymaltose infusions or multiple administrations of alternative preparations. The aim of the study was to confirm the safety and patient acceptance of ultrarapid iron polymaltose infusions as an alternative to slower treatments and ferric carboxymaltose.
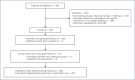
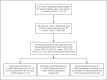
Method: An open-label, phase 4 safety study was conducted at a tertiary hospital, with consenting participants diagnosed with iron deficiency and requiring iron polymaltose up to 1,500 mg receiving the infusion over 15 min. The acute adverse event (AE) rates and their severities were compared to historical controls of 1- and 4-h iron polymaltose infusions from a retrospective study of 648 patients from the same study site. Delayed AEs as well as participant infusion acceptability were also studied.
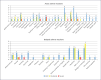
Results: Three hundred participants over a 2-year period received ultrarapid infusions of iron polymaltose with an acute AE rate of 18.7% and severe AE rate of 1.0%. The total and mild infusion AE rates were higher compared to those of slower infusions (p < 0.001), but comparable for moderate and severe AEs. Delayed reactions occurred in 12.5% of participants, with over 95% of them preferring repeat ultrarapid infusions if required again.
Conclusion: Iron polymaltose can be safely infused at ultrarapid rates when compared to slower infusions, with similar safety to ferric carboxymaltose, offering greater convenience for patients and reduced healthcare costs.
Introdução: A deficiência de ferro é uma condição comum, especialmente nos doentes com insuficiência renal e cardíaca e doença inflamatória intestinal. O ferro intravenoso é o método de tratamento preferido nestes doentes, mas normalmente requer infusões prolongadas ferropolimaltose ou múltiplas administrações de preparações alternativas. O objectivo deste estudo foi confirmar a segurança e a aceitação das infusões de ferro polimaltose ultrarápidas como alternativa às infusões mais lentas e à carboximaltose férrica.
Métodos: Estudo de segurança aberto, fase 4, num hospital terciário, incluindo doentes com ferropenia com necessidades de ferro-polimaltose até 1500 mg, que receberam a infusão durante 15 minutos. As taxas de eventos adversos (AE) agudos e as suas gravidades foram comparadas com controlos históricos de infusões de ferro-polimaltose de uma e quatro horas de duração, a partir de um estudo retrospectivo de 648 pacientes do mesmo centro. Foram também avaliados os EA diferidos, bem como a aceitabilidade da infusão dos participantes.
Resultados: Trezentos participantes receberam infusões ultrarápidas de ferro-polimaltose durante um período de 2 anos, com uma taxa de EA agudos de 18,7%, e uma taxa de EA graves de 1,0%. As taxas globais de AE e de AE ligeiros foram superiores às da infusão lenta (p < 0,001), mas comparável para os AEs moderados e graves. Reações tardias ocorreram em 12,5% dos participantes. Mais de 95% deles manifestaram preferência por repetir as infusões ultrarápidas, se necessidade subsequente de terapêutica.
Conclusão: A infusão ultra-rápida de ferro-polimaltose é segura quando comparada com infusões mais lentas, com segurança também semelhante à carboximaltose férrica, oferecendo maior comodidade e menores custos de saúde.
Keywords: Iron deficiency; Iron infusion; Safety; Treatment and hospital.
Copyright © 2023 by The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare. Iouri Banakh has previously received a research grant from the Society of Hospital Pharmacists of Australia for an unrelated study and conference attendance support from DBL.
Figures
References
-
- Pasricha SR, Flecknoe-Brown SC, Allen KJ, Gibson PR, McMahon LP, Olynyk JK, et al. Diagnosis and management of iron deficiency anaemia: a clinical update. Med J Aust. 2010;193((9)):525–532. - PubMed
-
- Garg M, Morrison G, Friedman A, Lau A, Lau D, Gibson PR. A rapid infusion protocol is safe for total dose iron polymaltose: time for change. Intern Med J. 2011;41((7)):548–554. - PubMed
-
- Banakh I, Lam A, Turek M, Htet TD, Vorlander C. Rapid versus standard iron polymaltose infusions: a single centre safety study. J Pharm Pract Res. 2017;47((2)):103–109.
-
- Newnham E, Ahmad I, Thornton A, Gibson PR. Safety of iron polymaltose given as a total dose iron infusion. Intern Med J. 2006;36((10)):672–674. - PubMed
LinkOut - more resources
Full Text Sources