The AsiDNA™ decoy mimicking DSBs protects the normal tissue from radiation toxicity through a DNA-PK/p53/p21-dependent G1/S arrest
- PMID: 38476631
- PMCID: PMC10928987
- DOI: 10.1093/narcan/zcae011
The AsiDNA™ decoy mimicking DSBs protects the normal tissue from radiation toxicity through a DNA-PK/p53/p21-dependent G1/S arrest
Abstract
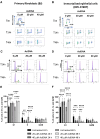
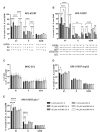
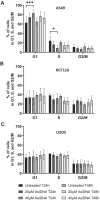
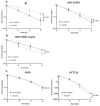
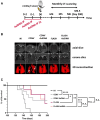
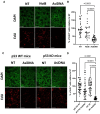
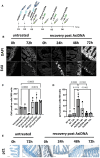
AsiDNA™, a cholesterol-coupled oligonucleotide mimicking double-stranded DNA breaks, was developed to sensitize tumour cells to radio- and chemotherapy. This drug acts as a decoy hijacking the DNA damage response. Previous studies have demonstrated that standalone AsiDNA™ administration is well tolerated with no additional adverse effects when combined with chemo- and/or radiotherapy. The lack of normal tissue complication encouraged further examination into the role of AsiDNA™ in normal cells. This research demonstrates the radioprotective properties of AsiDNA™. In vitro, AsiDNA™ induces a DNA-PK/p53/p21-dependent G1/S arrest in normal epithelial cells and fibroblasts that is absent in p53 deficient and proficient tumour cells. This cell cycle arrest improved survival after irradiation only in p53 proficient normal cells. Combined administration of AsiDNA™ with conventional radiotherapy in mouse models of late and early radiation toxicity resulted in decreased onset of lung fibrosis and increased intestinal crypt survival. Similar results were observed following FLASH radiotherapy in standalone or combined with AsiDNA™. Mechanisms comparable to those identified in vitro were detected both in vivo, in the intestine and ex vivo, in precision cut lung slices. Collectively, the results suggest that AsiDNA™ can partially protect healthy tissues from radiation toxicity by triggering a G1/S arrest in normal cells.
© The Author(s) 2024. Published by Oxford University Press on behalf of NAR Cancer.
Conflict of interest statement
Wael Jdey is employed by Valerio Therapeutics (former ONXEO). Valerio Therapeutics owns the patent for AsiDNA.
Figures



Similar articles
-
Role of p53 in the progression from ochratoxin A-induced DNA damage to gene mutations in the kidneys of mice.Toxicol Sci. 2015 Mar;144(1):65-76. doi: 10.1093/toxsci/kfu267. Epub 2015 Jan 29. Toxicol Sci. 2015. PMID: 25636497
-
Widdrol activates DNA damage checkpoint through the signaling Chk2-p53-Cdc25A-p21-MCM4 pathway in HT29 cells.Mol Cell Biochem. 2012 Apr;363(1-2):281-9. doi: 10.1007/s11010-011-1180-z. Epub 2011 Dec 11. Mol Cell Biochem. 2012. PMID: 22160829
-
Inhibition of DNA Repair by Inappropriate Activation of ATM, PARP, and DNA-PK with the Drug Agonist AsiDNA.Cells. 2022 Jul 8;11(14):2149. doi: 10.3390/cells11142149. Cells. 2022. PMID: 35883597 Free PMC article.
-
[Cell cycle regulation after exposure to ionizing radiation].Bull Cancer. 1999 Apr;86(4):345-57. Bull Cancer. 1999. PMID: 10341340 Review. French.
-
Cell cycle checkpoints and DNA repair preserve the stability of the human genome.Cancer Metastasis Rev. 1995 Mar;14(1):31-41. doi: 10.1007/BF00690209. Cancer Metastasis Rev. 1995. PMID: 7606819 Review.
Cited by
-
Amphiphilic Oligonucleotide Derivatives as a Tool to Study DNA Repair Proteins.Int J Mol Sci. 2025 Jul 23;26(15):7078. doi: 10.3390/ijms26157078. Int J Mol Sci. 2025. PMID: 40806211 Free PMC article.
-
Key molecular DNA damage responses of human cells to radiation.Front Cell Dev Biol. 2024 Jul 10;12:1422520. doi: 10.3389/fcell.2024.1422520. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39050891 Free PMC article. Review.
-
Mechanisms of the FLASH effect: current insights and advances.Front Cell Dev Biol. 2025 May 9;13:1575678. doi: 10.3389/fcell.2025.1575678. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40417178 Free PMC article. Review.
References
-
- De Ruysscher D., Niedermann G., Burnet N.G., Siva S., Lee A.W.M., Hegi-Johnson F. Radiotherapy toxicity. Nat. Rev. Dis. Primers. 2019; 5:13. - PubMed
-
- Hellman S. Improving the therapeutic index in breast cancer treatment: the Richard and Hinda Rosenthal Foundation Award lecture. Cancer Res. 1980; 40:4335–4342. - PubMed
-
- Quanz M., Berthault N., Roulin C., Roy M., Herbette A., Agrario C., Alberti C., Josserand V., Coll J.L., Sastre-Garau X. et al. . Small-molecule drugs mimicking DNA damage: a new strategy for sensitizing tumors to radiotherapy. Clin. Cancer Res. 2009; 15:1308–1316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

