Commercial hydrogel product for drug delivery based on route of administration
- PMID: 38476651
- PMCID: PMC10927762
- DOI: 10.3389/fchem.2024.1336717
Commercial hydrogel product for drug delivery based on route of administration
Abstract
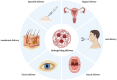
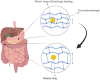
Hydrogels are hydrophilic, three-dimensional, cross-linked polymers that absorb significant amounts of biological fluids or water. Hydrogels possess several favorable properties, including flexibility, stimulus-responsiveness, versatility, and structural composition. They can be categorized according to their sources, synthesis route, response to stimulus, and application. Controlling the cross-link density matrix and the hydrogels' attraction to water while they're swelling makes it easy to change their porous structure, which makes them ideal for drug delivery. Hydrogel in drug delivery can be achieved by various routes involving injectable, oral, buccal, vaginal, ocular, and transdermal administration routes. The hydrogel market is expected to grow from its 2019 valuation of USD 22.1 billion to USD 31.4 billion by 2027. Commercial hydrogels are helpful for various drug delivery applications, such as transdermal patches with controlled release characteristics, stimuli-responsive hydrogels for oral administration, and localized delivery via parenteral means. Here, we are mainly focused on the commercial hydrogel products used for drug delivery based on the described route of administration.
Keywords: buccal; commercial products; drug delivery; hydrogel; oral; routes of administration; transdermal; vaginal.
Copyright © 2024 Raeisi and Farjadian.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Recent patents on stimuli responsive hydrogel drug delivery system.Recent Pat Drug Deliv Formul. 2013 Dec;7(3):206-15. doi: 10.2174/1872211307666131118141600. Recent Pat Drug Deliv Formul. 2013. PMID: 24237032 Review.
-
Synthesis and Characterization of Novel pH-Responsive Aminated Alginate Derivatives Hydrogels for Tissue Engineering and Drug Delivery.Curr Org Synth. 2023 Nov 3. doi: 10.2174/0115701794210967231016055949. Online ahead of print. Curr Org Synth. 2023. PMID: 37936472
-
Advances in Hydrogel-Based Drug Delivery Systems.Gels. 2024 Apr 13;10(4):262. doi: 10.3390/gels10040262. Gels. 2024. PMID: 38667681 Free PMC article. Review.
-
Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management.Pharmaceutics. 2021 Mar 8;13(3):357. doi: 10.3390/pharmaceutics13030357. Pharmaceutics. 2021. PMID: 33800402 Free PMC article. Review.
-
Synthesis, classification and properties of hydrogels: their applications in drug delivery and agriculture.J Mater Chem B. 2022 Jan 5;10(2):170-203. doi: 10.1039/d1tb01345a. J Mater Chem B. 2022. PMID: 34889937 Review.
Cited by
-
Injectable Biopolymer-Based Hydrogels: A Next-Generation Platform for Minimally Invasive Therapeutics.Gels. 2025 May 23;11(6):383. doi: 10.3390/gels11060383. Gels. 2025. PMID: 40558682 Free PMC article. Review.
-
Advanced Application of Polymer Nanocarriers in Delivery of Active Ingredients from Traditional Chinese Medicines.Molecules. 2024 Jul 26;29(15):3520. doi: 10.3390/molecules29153520. Molecules. 2024. PMID: 39124924 Free PMC article. Review.
-
Novel Buccal Xanthan Gum-Hyaluronic Acid Eutectogels with Dual Anti-Inflammatory and Antimicrobial Properties.Gels. 2025 Mar 15;11(3):208. doi: 10.3390/gels11030208. Gels. 2025. PMID: 40136913 Free PMC article.
-
Hydrogel-based biomaterials for brain regeneration after stroke: Gap to clinical translation.Biomater Transl. 2025 Jun 25;6(2):165-180. doi: 10.12336/bmt.24.00020. eCollection 2025. Biomater Transl. 2025. PMID: 40641992 Free PMC article. Review.
-
Enhanced Antifungal Efficacy through Controlled Delivery of Amphotericin B Loaded in Polyetheramine-Epoxide Nanogels.ACS Polym Au. 2025 Jul 25;5(4):406-419. doi: 10.1021/acspolymersau.5c00037. eCollection 2025 Aug 13. ACS Polym Au. 2025. PMID: 40821873 Free PMC article.
References
-
- Akbarian M., Chen S. H., Kianpour M., Farjadian F., Tayebi L., Uversky V. N. (2022). A review on biofilms and the currently available antibiofilm approaches: matrix-destabilizing hydrolases and anti-bacterial peptides as promising candidates for the food industries. Int. J. Biol. Macromol. 219, 1163–1179. 10.1016/j.ijbiomac.2022.08.192 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

