Untangling biodiversity interactions: A meta network on pollination in Earth's most diverse tropical savanna
- PMID: 38476698
- PMCID: PMC10928258
- DOI: 10.1002/ece3.11094
Untangling biodiversity interactions: A meta network on pollination in Earth's most diverse tropical savanna
Abstract
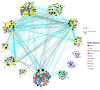
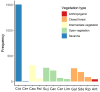
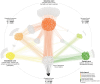
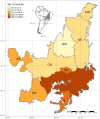
Pollination is vital for ecosystem functioning, especially in biodiversity-rich regions like the Brazilian Cerrado. Our research establishes a comprehensive meta network of pollinator-plant interactions within this biome. We quantified the importance of different pollinator groups, identifying keystone species. We examined potential biases in sampling effort and the spatial behavior of interactions within the heterogeneous Cerrado plant physiognomies. Our investigation uncovered 1499 interactions among 293 plant species and 386 visitor species, with legitimate pollination accounting for 42.4% of the interactions. The network exhibited modularity, driven by bees and insects, with vertebrates bridging diurnal and nocturnal modules. While a generalized pattern emerged, high specialization existed within modules due to habitat diversity. Bees, particularly Apis mellifera (exotic) and Trigona spinipes (native), played central roles as network hubs. Hummingbirds and bats, engaged in specialized interactions showing strong connectivity within and between modules. Interestingly, invertebrate-vertebrate modules were more prevalent than expected in the meta network. However, a bias was evident, primarily within specific biogeographical districts with fragmented landscapes and intrusion from other biomes. Variations in plant species and endemism rates influenced pollinator occurrence and the Cerrado network topology. Our study offers valuable insights into pollinator-plant interactions within the Cerrado, encompassing both invertebrates and vertebrates. The modeled network represents a significant step in understanding the structural complexity of pollination networks, integrating partial networks from diverse pollination systems within heterogeneous habitats. Nevertheless, a biogeographical bias could limit a comprehensive understanding of network functionality across the Cerrado.
Keywords: Brazilian Cerrado; conservation hotspot; floral visitors; modularity; mutualistic network; trait matching; zoophilia.
© 2024 The Authors. Ecology and Evolution published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest.
Figures

References
-
- Assunção, R. M. , Camargo, N. F. , Souza, L. S. , Rocha, E. M. , Tostes, G. M. , Sujii, E. R. , Pires, C. S. S. , & Togni, P. H. B. (2022). Landscape conservation and local interactions with non‐crop plants aid in structuring bee assemblages in organic tropical agroecosystems. Journal of Insect Conservation, 26(6), 933–945. 10.1007/s10841-022-00438-8 - DOI
-
- Báez‐Lizarazo, M. R. , Eggers, L. , Aguiar, A. J. C. , & Chauveau, O. (2021). Contrasting patterns of plant–pollinator interactions among four oil‐secreting species of Iridaceae from Pampean and Cerrado provinces (Brazil). Botanical Journal of the Linnean Society, 196(2), 256–277. 10.1093/botlinnean/boaa104 - DOI
-
- Bascompte, J. , & Jordano, P. (2007). Plant‐animal mutualistic networks: The architecture of biodiversity. Annual Review of Ecology, Evolution, and Systematics, 38(1), 567–593. 10.1146/annurev.ecolsys.38.091206.095818 - DOI
LinkOut - more resources
Full Text Sources

