Future perspective for the application of predictive biomarker testing in advanced stage non-small cell lung cancer
- PMID: 38476751
- PMCID: PMC10928270
- DOI: 10.1016/j.lanepe.2024.100839
Future perspective for the application of predictive biomarker testing in advanced stage non-small cell lung cancer
Abstract
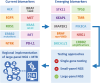
For patients with advanced stage non-small cell lung cancer (NSCLC), treatment strategies have changed significantly due to the introduction of targeted therapies and immunotherapy. In the last few years, we have seen an explosive growth of newly introduced targeted therapies in oncology and this development is expected to continue in the future. Besides primary targetable aberrations, emerging diagnostic biomarkers also include relevant co-occurring mutations and resistance mechanisms involved in disease progression, that have impact on optimal treatment management. To accommodate testing of pending biomarkers, it is necessary to establish routine large-panel next-generation sequencing (NGS) for all patients with advanced stage NSCLC. For cost-effectiveness and accessibility, it is recommended to implement predictive molecular testing using large-panel NGS in a dedicated, centralized expert laboratory within a regional oncology network. The central molecular testing center should host a regional Molecular Tumor Board and function as a hub for interpretation of rare and complex testing results and clinical decision-making.
Keywords: Next-generation sequencing; Non-small cell lung cancer; Predictive biomarker testing.
© 2024 The Authors.
Conflict of interest statement
WT has received consulting fees from Merck Sharp and Dohme, Bristol-Myers Squibb, and Altana (fees to institution), is board member of Dutch Society of Pathology and member of Council for Research and Innovation of the Federation of Medical Specialists (FMS); AB has received consulting fees from Sanofi (OncoCollective advisory board), payments or honoraria from Roche (oral presentation), support for attending ASCO 2023 from Pfizer; JB has received research grants from Amgen and Bristol-Myers Squibb, lecture honoraria from AstraZeneca, Merck Sharp and Dohme, Roche, Pfizer, Bristol-Myers Squibb, Boehringer Ingelheim, Novartis, GSK, Eli Lilly, Amgen, and Sanofi, and support for attending meetings and/or travel from Amgen; LB has received grants or contract from Takeda, Roche, AstraZeneca, and Bristol-Myers Squibb, payments or honoraria from Invitae, Eli Lilly, AstraZeneca, Roche, Merck Sharp and Dohme, Merck, Bristol-Myers Squibb, Pfizer, Novartis, Takeda, and Janssen, has participated in data safety monitoring boards or advisory boards for Invitae, Eli Lilly, AstraZeneca, Roche, Merck Sharp and Dohme, Merck, Bristol-Myers Squibb, Pfizer, Novartis, Takeda, and Janssen, is Int. Secretary of the Austrian Society of Pathology, PPS Membership and Awards Committee, and is Member of the Mesothelioma Committee of IASLC; RB has received payments or honoraria for lectures and advisory boards for Abbvie, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Boehringer-Ingelheim, Illumina, Janssen, Lilly, Merck-Serono, Merck Sharp and Dohme, Novartis, Qiagen, Pfizer, Roche, and Targos MP Inc., has received payments for expert testimony from Merck Sharp and Dohme, is co-founder and co-owner of Gnothis Inc (SE), Timer Therapeutics Inc (GE); MGOF has received fees for advisory boards participation, meetings, or as invited speaker, from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Lilly, Janssen, Merck Sharp and Dohme, Pfizer, Roche Farmacêutica Química Lda, and Takeda; MH has received payments or honoraria from Merck Sharp and Dohme, Roche, Lilly, AstraZeneca, and Takeda; PH has received consulting fees from AstraZeneca, Roche, Abbvie, Qiagen, and Pfizer, has received payments or honoraria from Thermofisher Scientist, Novartis, Janssen, AstraZeneca, and Biocartis, has received support for attending meetings and/or travel from Thermofisher Scientist, Novartis, Janssen, AstraZeneca, and Biocartis, has participated on a Data Safety Monitoring Board or Advisory Board for Thermofisher Scientist, Novartis, Janssen, AstraZeneca, Biocartis, Bristol-Myers Squibb, Sanofi, Roche, and Abbvie; LvK has received institutional grants or contract from Amgen, AstraZeneca, Bayer, Janssen-Cilag, Merck, Roche, and Servier, has received institutional payments or honoraria from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Eli Lilly, Novartis, Pfizer, and Roche, has received institutional support for attending meeting and/or travel from Roche, has participated on a Data Safety Monitoring Board or Advisory Board from Janssen-Cilag, Merck, and Roche, has a leadership or fiduciary role in the Commission Personalized Medicine – Belgium (unpaid), has stock or stock options in Cyclomics (institutional/personal); JCM has received payments or honoraria from Lilly Portugal, Novartis Portugal, Roche Portugal, Fresco Produções, Janssen-Cilag Portugal, Pierre Fabre Portugal, and Servier Portugal; has participated on a Data Safety Monitoring Board or Advisory Board of Roche Portugal; KM has received payments or honoraria from Takeda, Janssen, Pfizer, Merck Sharp and Dohme, Bristol-Myers Squibb, Roche, Amgen, Novartis, and Eli Lilly, has participated on a Data Safety Monitoring Board or Advisory Board of BMS, Takeda, AstraZeneca, Amgen, Roche, Boehringer Ingelheim, and Janssen; SP has received consulting fees (personal fees) from Amgen, AstraZeneca, Bayer, Blueprint, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, GSK, Guardant Health, Incyte, Janssen, Lilly, Merck Serono, Merck Sharp and Dohme, Novartis, Roche, Takeda, Pfizer, Seattle Genetics, Turning Point Therapeutics, and EQRx, has received payments or honoraria (personal fees) from AstraZeneca, Bayer, Guardant Health, Janssen, Merck Serono, Roche, and Takeda, has received payments for expert testimony (personal fees) from Roche, and Merck Serono, has received reimbursement of travel expenses from Janssen, and Roche, has participated on a Data Safety Monitoring Board or Advisory Board as per consulting fees, has a leadership role or fiduciary role (all unpaid) in the British Thoracic Oncology Group, ALK Positive UK, Lung Cancer Europe, Ruth Strauss Foundation, Mesothelioma Applied Research Foundation, and ETOP-IBCSG Partners Foundation Board; AR has received consulting fees for participation on advisory boards of AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Amgen, Merck Sharp and Dohme, Gilead, and Sanofi, has received honoraria for lectures and presentations from AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Amgen, Merck Sharp and Dohme, Gilead, Roche, Merck-Serono, has received support for attendings meetings and travel from Sanofi, Gilead, Roche, and Bristol-Myers Squibb; JW has received payments or honoraria, has received support for attendings meetings and travel, and has participated in a Data Safety Monitoring Board or Advisory Board, from Amgen, AstraZeneca, Bayer, Blueprint, BMS, Boehringer-Ingelheim, Chugai, Daiichi Sankyo, Janssen, Lilly, Loxo, Merck, Mirati, MSD, Novartis, Nuvalent, Pfizer, Pierre-Fabre, Roche, Seattle Genetics, Takeda, and Turning Point; ES has received unrestricted grants (all paid to UMCG institution) from Abbott, Biocartis, AstraZeneca, Invitae/Archer, Bayer, Bio-Rad, Roche, Agena Bioscience, CC Diagnostics, MSD/MERCK, and Boehringer Ingelheim, has received consulting fees (all paid to UMCG institution) from MSD/Merck, AstraZeneca, Roche, Novartis, Bayer, BMS, Lilly, Amgen, Illumina, Agena Bioscience, CC Diagnostics, Janssen Cilag (Johnson & Johnson), Astellas Pharma, GSK, Sinnovisionlab, and Sysmex, has received payments or honoraria (all paid to UMCG institution) from Bio-Rad, Seracare, Roche, Biocartis, Lilly, Agena Bioscience, and Illumina, has received support for attending meetings and/or travel from BioRad, Biocartis, Ageno Sciences, and Illumina, is a board member for the Dutch Society of Pathology (unpaid), European Society of Pathology (unpaid), European Liquid Biopsy Society (unpaid), is a secretary/member of the advisory committee for assessment of molecular diagnostics (cieBOD) (honoraria paid to UMCG institution), is committee member of national guideline advisory (honoraria paid to UMCG institution); AvdW, has received grants or contracts from AstraZeneca, Boehringer-Ingelheim, Pfizer, Roche, and Takeda, has received consulting fees from AstraZeneca, Janssen, Lilly, Roche, and Takeda, has received payments or honoraria from AstraZeneca, BMS, Lilly, Pfizer, and Roche, has a leadership or fiduciary role in the oncology section NVALT, guideline committee NSCLC and CUP, dure geneesmiddelen committee NVALT and FMS. All other authors declare no competing interests.
Figures
References
-
- Hendriks L.E., Kerr K.M., Menis J., et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(4):339–357. - PubMed
-
- Ettinger D.S., Wood D.E., Aisner D.L., et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2022;20(5):497–530. - PubMed
-
- Singh N., Temin S., Baker S., Jr., et al. Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO living guideline. J Clin Oncol. 2022;40(28):3310–3322. - PubMed
Publication types
LinkOut - more resources
Full Text Sources