Protecting infants against RSV disease: an impact and cost-effectiveness comparison of long-acting monoclonal antibodies and maternal vaccination
- PMID: 38476752
- PMCID: PMC10928299
- DOI: 10.1016/j.lanepe.2023.100829
Protecting infants against RSV disease: an impact and cost-effectiveness comparison of long-acting monoclonal antibodies and maternal vaccination
Erratum in
-
Correction to "Protecting infants against RSV disease: an impact and cost-effectiveness comparison of long-acting monoclonal antibodies and maternal vaccination" [The Lancet Regional Health - Europe 38 (2024) 100829].Lancet Reg Health Eur. 2024 Sep 11;45:101073. doi: 10.1016/j.lanepe.2024.101073. eCollection 2024 Oct. Lancet Reg Health Eur. 2024. PMID: 39308777 Free PMC article.
Abstract
Background: Two new products for preventing Respiratory Syncytial Virus (RSV) in young children have been licensed: a single-dose long-acting monoclonal antibody (la-mAB) and a maternal vaccine (MV). To facilitate the selection of new RSV intervention programmes for large-scale implementation, this study provides an assessment to compare the costs of potential programmes with the health benefits accrued.
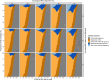
Methods: Using an existing dynamic transmission model, we compared maternal vaccination to la-mAB therapy against RSV in England and Wales by calculating the impact and cost-effectiveness. We calibrated a statistical model to the efficacy trial data to accurately capture their immune waning and estimated the impact of seasonal and year-round programmes for la-mAB and MV programmes. Using these impact estimates, we identified the most cost-effective programme across pricing and delivery cost assumptions.
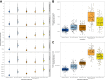
Findings: For infants under six months old in England and Wales, a year-round MV programme with 60% coverage would avert 32% (95% CrI 22-41%) of RSV hospital admissions and a year-round la-mAB programme with 90% coverage would avert 57% (95% CrI 41-69%). The MV programme has additional health benefits for pregnant women, which account for 20% of the population-level health burden averted. A seasonal la-mAB programme could be cost-effective for up to £84 for purchasing and administration (CCPA) and a seasonal MV could be cost-effective for up to £80 CCPA.
Interpretation: This modelling and cost-effectiveness analysis has shown that both the long-acting monoclonal antibodies and the maternal vaccine could substantially reduce the burden of RSV disease in the infant population. Our analysis has informed JCVI's recommendations for an RSV immunisation programme to protect newborns and infants.
Funding: National Institute for Health Research.
Keywords: Cost-effectiveness; Mathematical modelling; RSV; Vaccination.
© 2023 Published by Elsevier Ltd.
Conflict of interest statement
All authors declare no conflict of interest.
Figures



References
-
- Chapter G.B. 2015. Respiratory syncytial virus: the green book, [chapter 27]a.
-
- Kampmann B., Madhi S.A., Munjal I., et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388:1451–1464. - PubMed
-
- Hammitt L.L., Dagan R., Yuan Y., et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386:837–846. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

