Biochanin A inhibits endothelial dysfunction induced by IL‑6‑stimulated endothelial microparticles in Perthes disease via the NFκB pathway
- PMID: 38476892
- PMCID: PMC10928846
- DOI: 10.3892/etm.2024.12425
Biochanin A inhibits endothelial dysfunction induced by IL‑6‑stimulated endothelial microparticles in Perthes disease via the NFκB pathway
Abstract
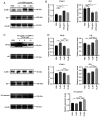
Endothelial dysfunction caused by the stimulation of endothelial microparticles (EMPs) by the inflammatory factor IL-6 is one of the pathogenic pathways associated with Perthes disease. The natural active product biochanin A (BCA) has an anti-inflammatory effect; however, whether it can alleviate endothelial dysfunction in Perthes disease is not known. The present in vitro experiments on human umbilical vein endothelial cells showed that 0-100 pg/ml IL-6-EMPs could induce endothelial dysfunction in a concentration-dependent manner, and the results of the Cell Counting Kit 8 assay revealed that, at concentrations of <20 µM, BCA had no cytotoxic effect. Reverse transcription-quantitative PCR demonstrated that BCA reduced the expression levels of the endothelial dysfunction indexes E-selectin and intercellular cell adhesion molecule-1 (ICAM-1) in a concentration-dependent manner. Immunofluorescence and western blotting illustrated that BCA increased the expression levels of zonula occludens-1 and decreased those of ICAM-1. Mechanistic studies showed that BCA inhibited activation of the NFκB pathway. In vivo experiments demonstrated that IL-6 was significantly increased in the rat model of ischemic necrosis of the femoral head, whereas BCA inhibited IL-6 production. Therefore, in Perthes disease, BCA may inhibit the NFκB pathway to suppress IL-6-EMP-induced endothelial dysfunction, and could thus be regarded as a potential treatment for Perthes disease.
Keywords: IL-6; NFκB signaling pathway; Perthes disease; biochanin A; endothelial dysfunction; endothelial microparticles.
Copyright: © 2024 Liu et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Increased circulating CD31+/CD42b-EMPs in Perthes disease and inhibit HUVECs angiogenesis via endothelial dysfunction.Life Sci. 2021 Jan 15;265:118749. doi: 10.1016/j.lfs.2020.118749. Epub 2020 Nov 18. Life Sci. 2021. PMID: 33220290
-
Prediction of the active compounds and mechanism of Biochanin A in the treatment of Legg-Calvé-Perthes disease based on network pharmacology and molecular docking.BMC Complement Med Ther. 2024 Jan 9;24(1):26. doi: 10.1186/s12906-023-04298-w. BMC Complement Med Ther. 2024. PMID: 38195507 Free PMC article.
-
Biochanin A inhibits lipopolysaccharide-induced inflammation in human umbilical vein endothelial cells.Life Sci. 2015 Sep 1;136:36-41. doi: 10.1016/j.lfs.2015.06.015. Epub 2015 Jul 2. Life Sci. 2015. PMID: 26141992
-
Endothelial microparticles reduce ICAM-1 expression in a microRNA-222-dependent mechanism.J Cell Mol Med. 2015 Sep;19(9):2202-14. doi: 10.1111/jcmm.12607. Epub 2015 Jun 17. J Cell Mol Med. 2015. PMID: 26081516 Free PMC article.
-
Biochanin A impedes STAT3 activation by upregulating p38δ MAPK phosphorylation in IL-6-stimulated macrophages.Inflamm Res. 2020 Nov;69(11):1143-1156. doi: 10.1007/s00011-020-01387-1. Epub 2020 Aug 27. Inflamm Res. 2020. PMID: 32852592
Cited by
-
Progress in understanding Legg-Calvé-Perthes disease etiology from a molecular and cellular biology perspective.Front Physiol. 2025 Feb 17;16:1514302. doi: 10.3389/fphys.2025.1514302. eCollection 2025. Front Physiol. 2025. PMID: 40041162 Free PMC article. Review.
-
Biochanin A enhances type H vessel formation and improves epiphysis deformities following ischemic osteonecrosis in juvenile mouse.Front Nutr. 2025 Jul 2;12:1583539. doi: 10.3389/fnut.2025.1583539. eCollection 2025. Front Nutr. 2025. PMID: 40672426 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous
