Predominant control of PDGF/PDGF receptor signaling in the migration and proliferation of human adipose‑derived stem cells under culture conditions with a combination of growth factors
- PMID: 38476902
- PMCID: PMC10928992
- DOI: 10.3892/etm.2024.12444
Predominant control of PDGF/PDGF receptor signaling in the migration and proliferation of human adipose‑derived stem cells under culture conditions with a combination of growth factors
Abstract
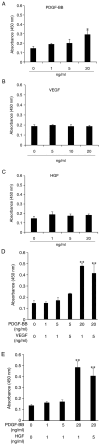
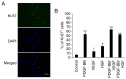
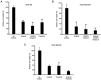
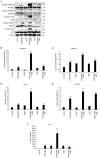
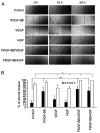
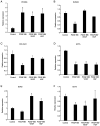
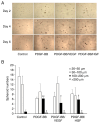
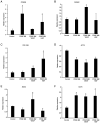
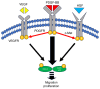
Human adipose-derived stem cells (hASCs) play important roles in regenerative medicine and tissue engineering. However, their clinical applications are limited because of their instability during cell culture. Platelet lysates (PLTs) contain large amounts of growth factors that are useful for manufacturing cellular products. Platelet-derived growth factor (PDGF) is a major growth factor in PLTs and a potent mitogen in hASCs. To optimize growth conditions, the effects of a combination of growth factors on the promotion of hASC proliferation were investigated. Moreover, PDGF-BB combined with vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) markedly enhanced the viability of hASCs compared with the effects of PDGF-BB alone. Neither VEGF nor HGF had any effect alone. All growth factor receptor inhibitors inhibited cell proliferation. Wound healing assays revealed that VEGF and HGF stimulated PDGF-dependent cell migration. The effects of these growth factors on the activation of their cognate receptors and signaling enzymes were assessed using immunoblotting. Phosphorylation of PDGF receptor (PDGFR)β, VEGF receptor (VEGFR)2 and MET proto-oncogene and receptor tyrosine kinase was induced by PDGF-BB treatment, and was further increased by treatment with PDGF-BB/VEGF and PDGF-BB/HGF. The levels of phospho-ERK1/2 and phospho-p38MAPK were increased by these treatments in parallel. Furthermore, the expression levels of SRY-box transcription factor 2 and peroxisome proliferator-activated receptor g were increased in PDGF-BB-treated cells, and PDGF-BB played a dominant role in spheroid formation. The findings of the present study highlighted that PDGF/PDGFR signaling played a predominant role in the proliferation and migration of hASCs, and suggested that PDGF was responsible for the efficacy of other growth factors when hASCs were cultured with PLTs.
Keywords: cell proliferation; hepatocyte growth factor; human adipose-derived stem cells; platelet-derived growth factor receptor; platelet-derived growth factor-BB; vascular endothelial growth factor.
Copyright: © 2024 Sun et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Gambogic acid induces G0/G1 cell cycle arrest and cell migration inhibition via suppressing PDGF receptor β tyrosine phosphorylation and Rac1 activity in rat aortic smooth muscle cells.J Atheroscler Thromb. 2010 Sep 30;17(9):901-13. doi: 10.5551/jat.3491. Epub 2010 Jun 11. J Atheroscler Thromb. 2010. PMID: 20543524
-
Platelet-rich plasma enhances the proliferation of human adipose stem cells through multiple signaling pathways.Stem Cell Res Ther. 2018 Apr 16;9(1):107. doi: 10.1186/s13287-018-0851-z. Stem Cell Res Ther. 2018. PMID: 29661222 Free PMC article.
-
Sodium butyrate inhibits platelet-derived growth factor-induced proliferation of vascular smooth muscle cells.Arterioscler Thromb Vasc Biol. 1995 Dec;15(12):2273-83. doi: 10.1161/01.atv.15.12.2273. Arterioscler Thromb Vasc Biol. 1995. PMID: 7489253
-
In vitro comparative evaluation of recombinant growth factors for tissue engineering of bladder in patients with neurogenic bladder.J Surg Res. 2014 Jan;186(1):63-72. doi: 10.1016/j.jss.2013.07.044. Epub 2013 Aug 15. J Surg Res. 2014. PMID: 24095026
-
Roles of PDGF/PDGFR signaling in various organs.Korean J Physiol Pharmacol. 2025 Mar 1;29(2):139-155. doi: 10.4196/kjpp.24.309. Epub 2024 Oct 31. Korean J Physiol Pharmacol. 2025. PMID: 39482238 Free PMC article. Review.
Cited by
-
Synergistic Effects of Insulin-like Growth Factor-1 and Platelet-Derived Growth Factor-BB in Tendon Healing.Int J Mol Sci. 2025 Apr 24;26(9):4039. doi: 10.3390/ijms26094039. Int J Mol Sci. 2025. PMID: 40362278 Free PMC article.
-
Molecular Insights into the Superiority of Platelet Lysate over FBS for hASC Expansion and Wound Healing.Cells. 2025 Jul 25;14(15):1154. doi: 10.3390/cells14151154. Cells. 2025. PMID: 40801587 Free PMC article.
-
Comparison of the efficacy of 12 interventions in the treatment of diabetic foot ulcers: a network meta-analysis.PeerJ. 2025 Aug 11;13:e19809. doi: 10.7717/peerj.19809. eCollection 2025. PeerJ. 2025. PMID: 40821981 Free PMC article.
-
Engraftment of human mesenchymal stem cells in a severely immunodeficient mouse.Inflamm Regen. 2024 Sep 26;44(1):40. doi: 10.1186/s41232-024-00353-2. Inflamm Regen. 2024. PMID: 39327616 Free PMC article.
-
Increasing the concentration of plasma molecules improves the biological activity of platelet-rich plasma for tissue regeneration.Sci Rep. 2025 Feb 6;15(1):4523. doi: 10.1038/s41598-025-88918-0. Sci Rep. 2025. PMID: 39915642 Free PMC article.
References
-
- Galeano-Garces C, Camilleri ET, Riester SM, Dudakovic A, Larson DR, Qu W, Smith J, Dietz AB, Im HJ, Krych AJ, et al. Molecular validation of chondrogenic differentiation and hypoxia responsiveness of platelet-lysate expanded adipose tissue-derived human mesenchymal stromal cells. Cartilage. 2017;8:283–299. doi: 10.1177/1947603516659344. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
