Fluorofenidone attenuates renal fibrosis by inhibiting lysosomal cathepsin‑mediated NLRP3 inflammasome activation
- PMID: 38476910
- PMCID: PMC10928820
- DOI: 10.3892/etm.2024.12430
Fluorofenidone attenuates renal fibrosis by inhibiting lysosomal cathepsin‑mediated NLRP3 inflammasome activation
Abstract
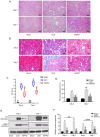
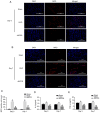
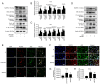
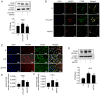
Currently, no antifibrotic drug in clinical use can effectively treat renal fibrosis. Fluorofenidone (AKFPD), a novel pyridone agent, significantly reduces renal fibrosis by inhibiting the activation of the NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome; however, the underlying mechanism of this inhibition is not fully understood. The present study aimed to reveal the molecular mechanism underlying the suppression of NLRP3 inflammasome activation by AKFPD. It investigated the effect of AKFPD on NLRP3 activation and lysosomal cathepsins in a unilateral ureteral obstruction (UUO) rat model, and hypoxia/reoxygenation (H/R)-treated HK-2 cells and murine peritoneal-derived macrophages (PDMs) stimulated with lipopolysaccharide (LPS) and ATP. The results confirmed that AKFPD suppressed renal interstitial fibrosis and inflammation by inhibiting NLRP3 inflammasome activation in UUO rat kidney tissues. In addition, AKFPD reduced the production of activated caspase-1 and maturation of IL-1β by suppressing NLRP3 inflammasome activation in H/R-treated HK-2 cells and murine PDMs stimulated with LPS and ATP. AKFPD also decreased the activities of cathepsins B, L and S both in vivo and in vitro. Notably, AKFPD downregulated cathepsin B expression and NLRP3 colocalization in the cytoplasm after lysosomal disruptions. Overall, the results suggested that AKFPD attenuates renal fibrosis by inhibiting lysosomal cathepsin-mediated activation of the NLRP3 inflammasome.
Keywords: antifibrotic therapy; fluorofenidone; inflammation; lysosomal cathepsin; renal fibrosis.
Copyright: © 2024 Zheng et al.
Conflict of interest statement
The authors declare they have no competing interests.
Figures



References
-
- Hackl MJ, Burford JL, Villanueva K, Lam L, Suszták K, Schermer B, Benzing T, Peti-Peterdi J. Tracking the fate of glomerular epithelial cells in vivo using serial multiphoton imaging in new mouse models with fluorescent lineage tags. Nat Med. 2013;19:1661–1666. doi: 10.1038/nm.3405. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
