A simple protocol for cultivating the bacterivorous soil nematode Caenorhabditis elegans in its natural ecology in the laboratory
- PMID: 38476935
- PMCID: PMC10929012
- DOI: 10.3389/fmicb.2024.1347797
A simple protocol for cultivating the bacterivorous soil nematode Caenorhabditis elegans in its natural ecology in the laboratory
Abstract
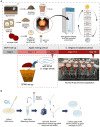
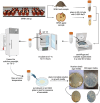
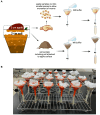
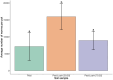
The complex interplay between an animal and its surrounding environment requires constant attentive observation in natural settings. Moreover, how ecological interactions are affected by an animal's genes is difficult to ascertain outside the laboratory. Genetic studies with the bacterivorous nematode Caenorhabditis elegans have elucidated numerous relationships between genes and functions, such as physiology, behaviors, and lifespan. However, these studies use standard laboratory culture that does not reflect C. elegans true ecology. C. elegans is found growing in nature and reproduced in large numbers in soils enriched with rotting fruit or vegetation, a source of abundant and diverse microbes that nourish the thriving populations of nematodes. We developed a simple mesocosm we call soil-fruit-natural-habitat that simulates the natural ecology of C. elegans in the laboratory. Apples were placed on autoclaved potted soils, and after a soil microbial solution was added, the mesocosm was subjected to day-night, temperature, and humidity cycling inside a growth chamber. After a period of apple-rotting, C elegans were added, and the growing worm population was observed. We determined optimal conditions for the growth of C. elegans and then performed an ecological succession experiment observing worm populations every few days. Our data showed that the mesocosm allows abundant growth and reproduction of C. elegans that resembles populations of the nematode found in rotting fruit in nature. Overall, our study presents a simple protocol that allows the cultivation of C. elegans in a natural habitat in the laboratory for a broad group of scientists to study various aspects of animal and microbial ecology.
Keywords: C. elegans; bacterial predator; ecological succession; nematode cultivation; nematode ecology; soil ecology; soil microbe.
Copyright © 2024 Indong, Park, Hong, Lyou, Han, Hong, Lee and Lee.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Comparison of Life Traits in Two Bacterivorous Nematodes Suggest Different Ecological Strategies to Exploit Similar Habitats.Life (Basel). 2022 Sep 28;12(10):1516. doi: 10.3390/life12101516. Life (Basel). 2022. PMID: 36294952 Free PMC article.
-
NGT-3D: a simple nematode cultivation system to study Caenorhabditis elegans biology in 3D.Biol Open. 2016 Apr 15;5(4):529-34. doi: 10.1242/bio.015743. Biol Open. 2016. PMID: 26962047 Free PMC article.
-
The prevalence of Caenorhabditis elegans across 1.5 years in selected North German locations: the importance of substrate type, abiotic parameters, and Caenorhabditis competitors.BMC Ecol. 2014 Feb 6;14:4. doi: 10.1186/1472-6785-14-4. BMC Ecol. 2014. PMID: 24502455 Free PMC article.
-
Making "Sense" of Ecology from a Genetic Perspective: Caenorhabditis elegans, Microbes and Behavior.Metabolites. 2022 Nov 9;12(11):1084. doi: 10.3390/metabo12111084. Metabolites. 2022. PMID: 36355167 Free PMC article. Review.
-
The Natural Biotic Environment of Caenorhabditis elegans.Genetics. 2017 May;206(1):55-86. doi: 10.1534/genetics.116.195511. Genetics. 2017. PMID: 28476862 Free PMC article. Review.
Cited by
-
Low humidity enhances thermotolerance in Caenorhabditis elegans.MicroPubl Biol. 2024 Nov 23;2024:10.17912/micropub.biology.001404. doi: 10.17912/micropub.biology.001404. eCollection 2024. MicroPubl Biol. 2024. PMID: 39650082 Free PMC article.
References
-
- Baker K. L., Langenheder S., Nicol G. W., Ricketts D., Killham K., Campbell C. D., et al. . (2009). Environmental and spatial characterisation of bacterial community composition in soil to inform sampling strategies. Soil Biol. Biochem. 41, 2292–2298. doi: 10.1016/j.soilbio.2009.08.010 - DOI
-
- Bennett A., Characteristics of different soils (2015), Available at: https://ahdb.org.uk
LinkOut - more resources
Full Text Sources
Miscellaneous

