Enhanced Bacterial Cuproptosis-Like Death via Reversal of Hypoxia Microenvironment for Biofilm Infection Treatment
- PMID: 38477452
- PMCID: PMC11109650
- DOI: 10.1002/advs.202308850
Enhanced Bacterial Cuproptosis-Like Death via Reversal of Hypoxia Microenvironment for Biofilm Infection Treatment
Abstract
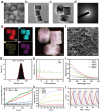
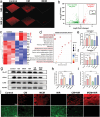
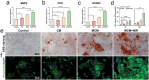
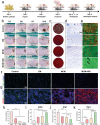
A recently emerging cell death pathway, known as copper-induced cell death, has demonstrated significant potential for treating infections. Existing research suggests that cells utilizing aerobic respiration, as opposed to those reliant on glycolysis, exhibit greater sensitivity to copper-induced death. Herein, a MnO2-loaded copper metal-organic frameworks platform is developed denoted as MCM, to enhance bacterial cuproptosis-like death via the remodeling of bacterial respiratory metabolism. The reversal of hypoxic microenvironments induced a cascade of responses, encompassing the reactivation of suppressed immune responses and the promotion of osteogenesis and angiogenesis. Initially, MCM catalyzed O2 production, alleviating hypoxia within the biofilm and inducing a transition in bacterial respiration mode from glycolysis to aerobic respiration. Subsequently, the sensitized bacteria, characterized by enhanced tricarboxylic acid cycle activity, underwent cuproptosis-like death owing to increased copper concentrations and aggregated intracellular dihydrolipoamide S-acetyltransferase (DLAT). The disruption of hypoxia also stimulated suppressed dendritic cells and macrophages, thereby strengthening their antimicrobial activity through chemotaxis and phagocytosis. Moreover, the nutritional effects of copper elements, coupled with hypoxia alleviation, synergistically facilitated the regeneration of bones and blood vessels. Overall, reshaping the infection microenvironment to enhance cuproptosis-like cell death presents a promising avenue for eradicating biofilms.
Keywords: biofilm; enhanced cuproptosis‐like death; hypoxia microenvironment; immunomodulation; respiratory metabolism.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- a) Zhu W., Mei J., Zhang X., Zhou J., Xu D., Su Z., Fang S., Wang J., Zhang X., Zhu C., Adv. Mater. 2022, 34, 2207961; - PubMed
- b) Wang J., Wang L., Pan J., Zhao J., Tang J., Jiang D., Hu P., Jia W., Shi J., Adv. Sci. 2021, 8, 2004010; - PMC - PubMed
- c) Wang G., Feng H., Hu L., Jin W., Hao Q., Gao A., Peng X., Li W., Wong K. Y., Wang H., Li Z., Chu P. K., Nat. Commun. 2018, 9, 2055. - PMC - PubMed
-
- a) Hou S., Mahadevegowda S. H., Lu D., Zhang K., Chan‐Park M. B., Duan H., Small 2020, 17, e2006357; - PubMed
- b) Chang M., Hou Z., Wang M., Li C., Lin J., Adv. Mater. 2020, 33, 2004788; - PubMed
- c) Xie Y., Liu Y., Yang J., Liu Y., Hu F., Zhu K., Jiang X., Angew. Chem. Int. Ed. 2018, 57, 3958; - PubMed
- d) Zhao H., Zhao C., Liu Z., Yi J., Liu X., Ren J., Qu X., Angew. Chem. Int. Ed. 2023, 62, e202303989. - PubMed
MeSH terms
Substances
Grants and funding
- 81974340/National Natural Science Foundation of China
- 82172455/National Natural Science Foundation of China
- YG2021ZD22/Interdisciplinary Program of Shanghai Jiao Tong University
- SHDC12021117/Shanghai Shenkang Hospital Development Center Clinical Innovation Project
- 2023M732289/China Postdoctoral Science Foundation
LinkOut - more resources
Full Text Sources
