Toward the development of a molecular toolkit for the microbial remediation of per-and polyfluoroalkyl substances
- PMID: 38477530
- PMCID: PMC11022551
- DOI: 10.1128/aem.00157-24
Toward the development of a molecular toolkit for the microbial remediation of per-and polyfluoroalkyl substances
Abstract
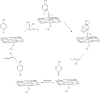
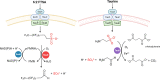
Per- and polyfluoroalkyl substances (PFAS) are highly fluorinated synthetic organic compounds that have been used extensively in various industries owing to their unique properties. The PFAS family encompasses diverse classes, with only a fraction being commercially relevant. These substances are found in the environment, including in water sources, soil, and wildlife, leading to human exposure and fueling concerns about potential human health impacts. Although PFAS degradation is challenging, biodegradation offers a promising, eco-friendly solution. Biodegradation has been effective for a variety of organic contaminants but is yet to be successful for PFAS due to a paucity of identified microbial species capable of transforming these compounds. Recent studies have investigated PFAS biotransformation and fluoride release; however, the number of specific microorganisms and enzymes with demonstrable activity with PFAS remains limited. This review discusses enzymes that could be used in PFAS metabolism, including haloacid dehalogenases, reductive dehalogenases, cytochromes P450, alkane and butane monooxygenases, peroxidases, laccases, desulfonases, and the mechanisms of microbial resistance to intracellular fluoride. Finally, we emphasize the potential of enzyme and microbial engineering to advance PFAS degradation strategies and provide insights for future research in this field.
Keywords: PFAS; defluorinase; defluorination; dehalogenase; microbial remediation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

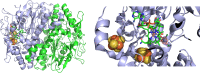
References
-
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SPJ. 2011. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 7:513–541. doi: 10.1002/ieam.258 - DOI - PMC - PubMed
-
- Williams AJ, Gaines LGT, Grulke CM, Lowe CN, Sinclair GFB, Samano V, Thillainadarajah I, Meyer B, Patlewicz G, Richard AM. 2022. Assembly and curation of lists of per- and polyfluoroalkyl substances (PFAS) to support environmental science research. Front Environ Sci 10:1–13. doi: 10.3389/fenvs.2022.850019 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

