A Multifunctional Therapeutic Strategy Using P7C3 as A Countermeasure Against Bone Loss and Fragility in An Ovariectomized Rat Model of Postmenopausal Osteoporosis
- PMID: 38477537
- PMCID: PMC11151083
- DOI: 10.1002/advs.202308698
A Multifunctional Therapeutic Strategy Using P7C3 as A Countermeasure Against Bone Loss and Fragility in An Ovariectomized Rat Model of Postmenopausal Osteoporosis
Abstract
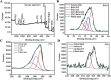
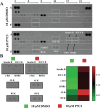
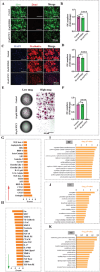
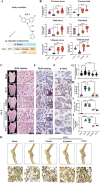
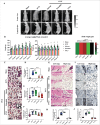
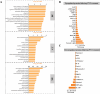
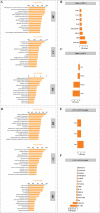
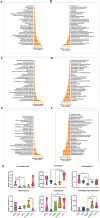
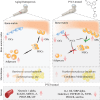
By 2060, an estimated one in four Americans will be elderly. Consequently, the prevalence of osteoporosis and fragility fractures will also increase. Presently, no available intervention definitively prevents or manages osteoporosis. This study explores whether Pool 7 Compound 3 (P7C3) reduces progressive bone loss and fragility following the onset of ovariectomy (OVX)-induced osteoporosis. Results confirm OVX-induced weakened, osteoporotic bone together with a significant gain in adipogenic body weight. Treatment with P7C3 significantly reduced osteoclastic activity, bone marrow adiposity, whole-body weight gain, and preserved bone area, architecture, and mechanical strength. Analyses reveal significantly upregulated platelet derived growth factor-BB and leukemia inhibitory factor, with downregulation of interleukin-1 R6, and receptor activator of nuclear factor kappa-B (RANK). Together, proteomic data suggest the targeting of several key regulators of inflammation, bone, and adipose turnover, via transforming growth factor-beta/SMAD, and Wingless-related integration site/be-catenin signaling pathways. To the best of the knowledge, this is first evidence of an intervention that drives against bone loss via RANK. Metatranscriptomic analyses of the gut microbiota show P7C3 increased Porphyromonadaceae bacterium, Candidatus Melainabacteria, and Ruminococcaceae bacterium abundance, potentially contributing to the favorable inflammatory, and adipo-osteogenic metabolic regulation observed. The results reveal an undiscovered, and multifunctional therapeutic strategy to prevent the pathological progression of OVX-induced bone loss.
Keywords: P7C3; bone loss; bone protection; fragility fracture; osteoporosis; ovariectomy.
© 2024 The Authors. Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
