CHD7 regulates craniofacial cartilage development via controlling HTR2B expression
- PMID: 38477756
- PMCID: PMC11262153
- DOI: 10.1093/jbmr/zjae024
CHD7 regulates craniofacial cartilage development via controlling HTR2B expression
Abstract
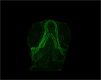
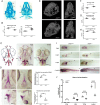
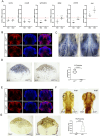
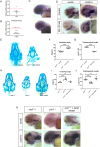
Mutations in the Chromodomain helicase DNA-binding protein 7 - coding gene (CHD7) cause CHARGE syndrome (CS). Although craniofacial and skeletal abnormalities are major features of CS patients, the role of CHD7 in bone and cartilage development remain largely unexplored. Here, using a zebrafish (Danio rerio) CS model, we show that chd7-/- larvae display abnormal craniofacial cartilage development and spinal deformities. The craniofacial and spine defects are accompanied by a marked reduction of bone mineralization. At the molecular level, we show that these phenotypes are associated with significant reduction in the expression levels of osteoblast differentiation markers. Additionally, we detected a marked depletion of collagen 2α1 in the cartilage of craniofacial regions and vertebrae, along with significantly reduced number of chondrocytes. Chondrogenesis defects are at least in part due to downregulation of htr2b, which we found to be also dysregulated in human cells derived from an individual with CHD7 mutation-positive CS. Overall, this study thus unveils an essential role for CHD7 in cartilage and bone development, with potential clinical relevance for the craniofacial defects associated with CS.
Keywords: cells of bone; diseases and disorders of/related to bone; genetic research.
Plain language summary
Patients with CHARGE syndrome exhibit skeletal defects. CHARGE syndrome is primarily caused by mutations in the chromatin remodeler-coding gene CHD7. To investigate the poorly characterized role of CHD7 in cartilage and bone development, here, we examine the craniofacial and bone anomalies in a zebrafish chd7-/- mutant model. We find that zebrafish mutant larvae exhibit striking dysmorphism of craniofacial structures and spinal deformities. Notably, we find a significant reduction in osteoblast, chondrocyte, and collagen matrix markers. This work provides important insights to improve our understanding of the role of chd7 in skeletal development.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Conflict of interest statement
Shunmoogum (Kessen) Patten is part of the Board of Directors of the International Zebrafish Society. The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

