Circadian patterns of behaviour change during pregnancy in mice
- PMID: 38477893
- PMCID: PMC11607885
- DOI: 10.1113/JP285553
Circadian patterns of behaviour change during pregnancy in mice
Abstract
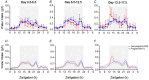
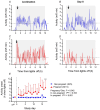
Food intake and activity adapt during pregnancy to meet the increased energy demands. In comparison to non-pregnant females, pregnant mice consume more food, eating larger meals during the light phase, and reduce physical activity. How pregnancy changes the circadian timing of behaviour was less clear. We therefore randomised female C57BL/6J mice to mating for study until early (n = 10), mid- (n = 10) or late pregnancy (n = 11) or as age-matched, non-pregnant controls (n = 12). Mice were housed individually in Promethion cages with a 12 h light-12 h dark cycle [lights on at 07.00 h, Zeitgeber (ZT)0] for behavioural analysis. Food intake between ZT10 and ZT11 was greater in pregnant than non-pregnant mice on days 6.5-12.5 and 12.5-17.5. In mice that exhibited a peak in the last 4 h of the light phase (ZT8-ZT12), peaks were delayed by 1.6 h in the pregnant compared with the non-pregnant group. Food intake immediately after dark-phase onset (ZT13-ZT14) was greater in the pregnant than non-pregnant group during days 12.5-17.5. Water intake patterns corresponded to food intake. From days 0.5-6.5 onwards, the pregnant group moved less during the dark phase, with decreased probability of being awake, in comparison to the non-pregnant group. The onset of dark-phase activity, peaks in activity, and wakefulness were all delayed during pregnancy. In conclusion, increased food intake during pregnancy reflects increased amplitude of eating behaviour, without longer duration. Decreases in activity also contribute to positive energy balance in pregnancy, with delays to all measured behaviours evident from mid-pregnancy onwards. KEY POINTS: Circadian rhythms synchronise daily behaviours including eating, drinking and sleep, but how these change in pregnancy is unclear. Food intake increased, with delays in peaks of food intake behaviour late in the light phase from days 6.5 to 12.5 of pregnancy, in comparison to the non-pregnant group. The onset of activity after lights off (dark phase) was delayed in pregnant compared with non-pregnant mice. Activity decreased by ∼70% in the pregnant group, particularly in the dark (active) phase, with delays in peaks of wakefulness evident from days 0.5-6.5 of pregnancy onwards. These behavioural changes contribute to positive energy balance during pregnancy. Delays in circadian behaviours during mouse pregnancy were time period and pregnancy stage specific, implying different regulatory mechanisms.
Keywords: circadian; food intake; physical activity; pregnancy.
© 2024 The Authors. The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Albers, E. E. , Gerall, A. A. , & Axelson, J. F. (1981). Effect of reproductive state on circadian periodicity in the rat. Physiology & Behavior, 26(1), 21–25. - PubMed
-
- Brooks, M. E. , Kristensen, K. , Benthem, K. J. V. , Magnusson, A. , Berg, C. W. , Nielsen, A. , Skaug, H. J. , Mächler, M. , & Bolker, B. M. (2017). glmmTMB balances speed and flexibility among packages for zero‐inflated generalized linear mixed modeling. R Journal, 9(2), 378–400.
-
- Bürkner, P.‐C. (2017). Brms : An R package for bayesian multilevel models using stan. Journal of Statistical Software, 80(1), 1–28.
-
- Cai, C. , Vandermeer, B. , Khurana, R. , Nerenberg, K. , Featherstone, R. , Sebastianski, M. , & Davenport, M. H. (2019). The impact of occupational shift work and working hours during pregnancy on health outcomes: A systematic review and meta‐analysis. American Journal of Obstetrics and Gynecology, 221(6), 563–576. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
