COVID-19 Rebound After VV116 vs Nirmatrelvir-Ritonavir Treatment: A Randomized Clinical Trial
- PMID: 38477921
- PMCID: PMC10938176
- DOI: 10.1001/jamanetworkopen.2024.1765
COVID-19 Rebound After VV116 vs Nirmatrelvir-Ritonavir Treatment: A Randomized Clinical Trial
Abstract
Importance: With the widespread use of anti-SARS-CoV-2 drugs, accumulating data have revealed potential viral load rebound after treatment.
Objective: To compare COVID-19 rebound after a standard 5-day course of antiviral treatment with VV116 vs nirmatrelvir-ritonavir.
Design, setting, and participants: This is a single-center, investigator-blinded, randomized clinical trial conducted in Shanghai, China. Adult patients with mild-to-moderate COVID-19 and within 5 days of SARS-CoV-2 infection were enrolled between December 20, 2022, and January 19, 2023, and randomly allocated to receive either VV116 or nirmatrelvir-ritonavir.
Interventions: Participants in the VV116 treatment group received oral 600-mg VV116 tablets every 12 hours on day 1 and 300 mg every 12 hours on days 2 through 5. Participants in the nirmatrelvir-ritonavir treatment group received oral nirmatrelvir-ritonavir tablets with 300 mg of nirmatrelvir plus 100 mg of ritonavir every 12 hours for 5 days. Participants were followed up every other day until day 28 and every week until day 60.
Main outcomes and measures: The primary outcome was viral load rebound (VLR), defined as a half-log increase in viral RNA copies per milliliter compared with treatment completion. Secondary outcomes included a reduction in the cycle threshold value of 1.5 or more, time until VLR, and symptom rebound, defined as an increase of more than 2 points in symptom score compared with treatment completion. The primary outcome and secondary outcomes were analyzed using the full analysis set. Sensitivity analyses were conducted using the per protocol set. Adverse events were analyzed using the safety analysis set.
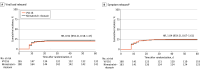
Results: The full analysis set included 345 participants (mean [SD] age, 53.2 [16.8] years; 175 [50.7%] were men) who received VV116 (n = 165) or nirmatrelvir-ritonavir (n = 180). Viral load rebound occurred in 33 patients (20.0%) in the VV116 group and 39 patients (21.7%) in the nirmatrelvir-ritonavir group (P = .70). Symptom rebound occurred in 41 of 160 patients (25.6%) in the VV116 group and 40 of 163 patients (24.5%) in the nirmatrelvir-ritonavir group (P = .82). Viral whole-genome sequencing of 24 rebound cases revealed the same lineage at baseline and at viral load rebound in each case.
Conclusions and relevance: In this randomized clinical trial of patients with mild-to-moderate COVID-19, viral load rebound and symptom rebound were both common after a standard 5-day course of treatment with either VV116 or nirmatrelvir-ritonavir. Prolongation of treatment duration might be investigated to reduce COVID-19 rebound.
Trial registration: Chinese Clinical Trial Registry Identifier: ChiCTR2200066811.
Conflict of interest statement
Figures
Similar articles
-
Modeling suggests SARS-CoV-2 rebound after nirmatrelvir-ritonavir treatment is driven by target cell preservation coupled with incomplete viral clearance.J Virol. 2025 Mar 18;99(3):e0162324. doi: 10.1128/jvi.01623-24. Epub 2025 Feb 4. J Virol. 2025. PMID: 39902924 Free PMC article.
-
VV116 versus Nirmatrelvir-Ritonavir for Oral Treatment of Covid-19.N Engl J Med. 2023 Feb 2;388(5):406-417. doi: 10.1056/NEJMoa2208822. Epub 2022 Dec 28. N Engl J Med. 2023. PMID: 36577095 Free PMC article. Clinical Trial.
-
Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19.N Engl J Med. 2022 Apr 14;386(15):1397-1408. doi: 10.1056/NEJMoa2118542. Epub 2022 Feb 16. N Engl J Med. 2022. PMID: 35172054 Free PMC article. Clinical Trial.
-
Coronavirus disease 2019 rebounds following nirmatrelvir/ritonavir treatment.J Med Virol. 2023 Feb;95(2):e28430. doi: 10.1002/jmv.28430. J Med Virol. 2023. PMID: 36571273 Free PMC article. Review.
-
The safety and efficacy of oral antiviral drug VV116 for treatment of COVID-19: A systematic review.Medicine (Baltimore). 2023 Jul 7;102(27):e34105. doi: 10.1097/MD.0000000000034105. Medicine (Baltimore). 2023. PMID: 37417593 Free PMC article.
Cited by
-
Modeling suggests SARS-CoV-2 rebound after nirmatrelvir-ritonavir treatment is driven by target cell preservation coupled with incomplete viral clearance.J Virol. 2025 Mar 18;99(3):e0162324. doi: 10.1128/jvi.01623-24. Epub 2025 Feb 4. J Virol. 2025. PMID: 39902924 Free PMC article.
-
Modeling suggests SARS-CoV-2 rebound after nirmatrelvir-ritonavir treatment is driven by target cell preservation coupled with incomplete viral clearance.bioRxiv [Preprint]. 2024 Sep 16:2024.09.13.613000. doi: 10.1101/2024.09.13.613000. bioRxiv. 2024. Update in: J Virol. 2025 Mar 18;99(3):e0162324. doi: 10.1128/jvi.01623-24. PMID: 39345409 Free PMC article. Updated. Preprint.
-
Drug treatments for mild or moderate covid-19: systematic review and network meta-analysis.BMJ. 2025 May 29;389:e081165. doi: 10.1136/bmj-2024-081165. BMJ. 2025. PMID: 40441732 Free PMC article.
-
Viral and Symptom Rebound Following Anti-Severe Acute Respiratory Syndrome Coronavirus 2 Monoclonal Antibody Therapy in a Randomized Placebo-Controlled Trial.J Infect Dis. 2025 Feb 4;231(1):131-136. doi: 10.1093/infdis/jiae501. J Infect Dis. 2025. PMID: 39400063 Clinical Trial.
-
Analysis of Clinical Criteria for Discharge Among Patients Hospitalized for COVID-19: Development and Validation of a Risk Prediction Model.J Gen Intern Med. 2024 Nov;39(14):2649-2661. doi: 10.1007/s11606-024-08856-x. Epub 2024 Jun 27. J Gen Intern Med. 2024. PMID: 38937368 Free PMC article.
References
-
- US Centers for Disease Control and Prevention . CDC Health Advisory: COVID-19 rebound after Paxlovid treatment. May 24, 2022. Accessed August 21, 2023. https://emergency.cdc.gov/han/2022/pdf/CDC_HAN_467.pdf
-
- Wang L, Berger NA, Davis PB, Kaelber DC, Volkow ND, Xu R. COVID-19 rebound after Paxlovid and molnupiravir during January-June 2022. medRxiv. Preprint posted online June 22, 2022. doi:10.1101/2022.06.21.22276724 - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

