Intraventricular CARv3-TEAM-E T Cells in Recurrent Glioblastoma
- PMID: 38477966
- PMCID: PMC11162836
- DOI: 10.1056/NEJMoa2314390
Intraventricular CARv3-TEAM-E T Cells in Recurrent Glioblastoma
Abstract
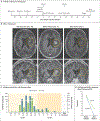
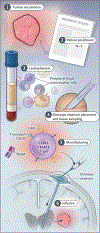
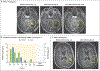
In this first-in-human, investigator-initiated, open-label study, three participants with recurrent glioblastoma were treated with CARv3-TEAM-E T cells, which are chimeric antigen receptor (CAR) T cells engineered to target the epidermal growth factor receptor (EGFR) variant III tumor-specific antigen, as well as the wild-type EGFR protein, through secretion of a T-cell-engaging antibody molecule (TEAM). Treatment with CARv3-TEAM-E T cells did not result in adverse events greater than grade 3 or dose-limiting toxic effects. Radiographic tumor regression was dramatic and rapid, occurring within days after receipt of a single intraventricular infusion, but the responses were transient in two of the three participants. (Funded by Gateway for Cancer Research and others; INCIPIENT ClinicalTrials.gov number, NCT05660369.).
Copyright © 2024 Massachusetts Medical Society.
Figures

Comment in
-
Infusion of CARv3-TEAM-E T Cells in Glioblastoma.N Engl J Med. 2024 Jun 27;390(24):2330. doi: 10.1056/NEJMc2405721. N Engl J Med. 2024. PMID: 38924745 No abstract available.
-
Infusion of CARv3-TEAM-E T Cells in Glioblastoma. Reply.N Engl J Med. 2024 Jun 27;390(24):2330-2331. doi: 10.1056/NEJMc2405721. N Engl J Med. 2024. PMID: 38924746 No abstract available.
References
-
- Choi BD, Yu X, Castano AP, et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat Biotechnol 2019;37:1049–58. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
