Carbon Nanomaterial Fluorescent Probes and Their Biological Applications
- PMID: 38478064
- PMCID: PMC10979413
- DOI: 10.1021/acs.chemrev.3c00581
Carbon Nanomaterial Fluorescent Probes and Their Biological Applications
Abstract
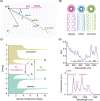
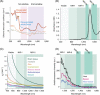
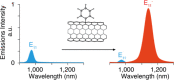
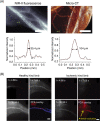
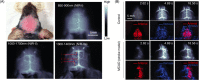
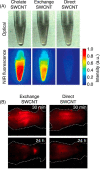
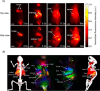
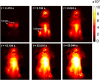
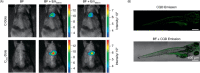
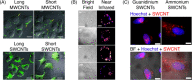
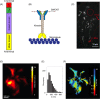
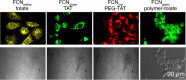
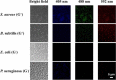
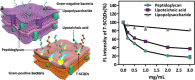
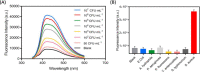
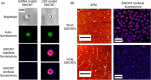
Fluorescent carbon nanomaterials have broadly useful chemical and photophysical attributes that are conducive to applications in biology. In this review, we focus on materials whose photophysics allow for the use of these materials in biomedical and environmental applications, with emphasis on imaging, biosensing, and cargo delivery. The review focuses primarily on graphitic carbon nanomaterials including graphene and its derivatives, carbon nanotubes, as well as carbon dots and carbon nanohoops. Recent advances in and future prospects of these fields are discussed at depth, and where appropriate, references to reviews pertaining to older literature are provided.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

















Similar articles
-
Aptamer-assembled nanomaterials for biosensing and biomedical applications.Small. 2011 Sep 5;7(17):2428-36. doi: 10.1002/smll.201100250. Epub 2011 Jul 4. Small. 2011. PMID: 21726041 Review.
-
Recent applications of carbon nanomaterials in fluorescence biosensing and bioimaging.Chem Commun (Camb). 2015 Jul 21;51(57):11346-58. doi: 10.1039/c5cc02887f. Chem Commun (Camb). 2015. PMID: 25990681 Review.
-
Using Nanomaterials as Excellent Immobilisation Layer for Biosensor Design.Biosensors (Basel). 2023 Jan 27;13(2):192. doi: 10.3390/bios13020192. Biosensors (Basel). 2023. PMID: 36831958 Free PMC article. Review.
-
Carbon nanomaterials: multi-functional agents for biomedical fluorescence and Raman imaging.Chem Soc Rev. 2015 Jul 21;44(14):4672-98. doi: 10.1039/c4cs00306c. Chem Soc Rev. 2015. PMID: 25406743 Review.
-
Progress and Prospects on the Fabrication of Graphene-Based Nanostructures for Energy Storage, Energy Conversion and Biomedical Applications.Chem Asian J. 2021 Jun 1;16(11):1365-1381. doi: 10.1002/asia.202100216. Epub 2021 May 7. Chem Asian J. 2021. PMID: 33899344 Review.
Cited by
-
Integrating Single-Walled Carbon Nanotubes into Supramolecular Assemblies: From Basic Interactions to Emerging Applications.ACS Nano. 2024 Oct 29;18(43):29380-29393. doi: 10.1021/acsnano.4c06843. Epub 2024 Oct 20. ACS Nano. 2024. PMID: 39428637 Free PMC article. Review.
-
Functionalization and solubilization of polycyclic aromatic compounds by sulfoniumization.Chem Sci. 2025 Apr 11;16(19):8262-8267. doi: 10.1039/d5sc01415h. eCollection 2025 May 14. Chem Sci. 2025. PMID: 40271021 Free PMC article.
-
Highly adaptable deep-learning platform for automated detection and analysis of vesicle exocytosis.Nat Commun. 2025 Jul 12;16(1):6450. doi: 10.1038/s41467-025-61579-3. Nat Commun. 2025. PMID: 40651941 Free PMC article.
-
Role of Oxygen Defects in Eliciting a Divergent Fluorescence Response of Single-Walled Carbon Nanotubes to Dopamine and Serotonin.ACS Nano. 2024 Dec 17;18(50):34134-34146. doi: 10.1021/acsnano.4c10360. Epub 2024 Dec 4. ACS Nano. 2024. PMID: 39632591 Free PMC article.
-
Interactional Fingerprints Offer Accessible, Rapid, and Qualitative Characterization of Graphene Oxide.J Am Chem Soc. 2025 Jul 23;147(29):25471-25477. doi: 10.1021/jacs.5c05355. Epub 2025 Jul 9. J Am Chem Soc. 2025. PMID: 40633108 Free PMC article.
References
-
- Weisman R. B.Fluorescence Spectroscopy of Single-Walled Carbon Nanotubes. Applied Physics of Carbon Nanotubes; Rotkin S. V., Subramoney S., Eds.; Springer, 2005; pp 183–202.10.1007/3-540-28075-8_7. - DOI
-
- Schöppler F.; et al. Molar Extinction Coefficient of Single-Wall Carbon Nanotubes. J. Phys. Chem. C 2011, 115, 14682–14686. 10.1021/jp205289h. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

