End-to-end deep learning approach to mouse behavior classification from cortex-wide calcium imaging
- PMID: 38478563
- PMCID: PMC10986998
- DOI: 10.1371/journal.pcbi.1011074
End-to-end deep learning approach to mouse behavior classification from cortex-wide calcium imaging
Abstract
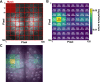
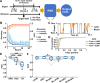
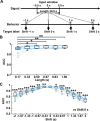
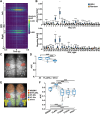
Deep learning is a powerful tool for neural decoding, broadly applied to systems neuroscience and clinical studies. Interpretable and transparent models that can explain neural decoding for intended behaviors are crucial to identifying essential features of deep learning decoders in brain activity. In this study, we examine the performance of deep learning to classify mouse behavioral states from mesoscopic cortex-wide calcium imaging data. Our convolutional neural network (CNN)-based end-to-end decoder combined with recurrent neural network (RNN) classifies the behavioral states with high accuracy and robustness to individual differences on temporal scales of sub-seconds. Using the CNN-RNN decoder, we identify that the forelimb and hindlimb areas in the somatosensory cortex significantly contribute to behavioral classification. Our findings imply that the end-to-end approach has the potential to be an interpretable deep learning method with unbiased visualization of critical brain regions.
Copyright: © 2024 Ajioka et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- LeCun Y, Bengio Y, Hinton G. Deep learning. nature. 2015. May 28;521(7553):436–44. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

