MLL1 regulates cytokine-driven cell migration and metastasis
- PMID: 38478601
- PMCID: PMC10936879
- DOI: 10.1126/sciadv.adk0785
MLL1 regulates cytokine-driven cell migration and metastasis
Abstract
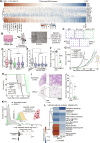
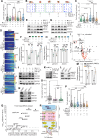
Cell migration is a critical contributor to metastasis. Cytokine production and its role in cancer cell migration have been traditionally associated with immune cells. We find that the histone methyltransferase Mixed-Lineage Leukemia 1 (MLL1) controls 3D cell migration via cytokines, IL-6, IL-8, and TGF-β1, secreted by the cancer cells themselves. MLL1, with its scaffold protein Menin, controls actin filament assembly via the IL-6/8/pSTAT3/Arp3 axis and myosin contractility via the TGF-β1/Gli2/ROCK1/2/pMLC2 axis, which together regulate dynamic protrusion generation and 3D cell migration. MLL1 also regulates cell proliferation via mitosis-based and cell cycle-related pathways. Mice bearing orthotopic MLL1-depleted tumors exhibit decreased lung metastatic burden and longer survival. MLL1 depletion leads to lower metastatic burden even when controlling for the difference in primary tumor growth rates. Combining MLL1-Menin inhibitor with paclitaxel abrogates tumor growth and metastasis, including preexistent metastasis. These results establish MLL1 as a potent regulator of cell migration and highlight the potential of targeting MLL1 in patients with metastatic disease.
Figures





Similar articles
-
The menin-MLL1 interaction is a molecular dependency in NUP98-rearranged AML.Blood. 2022 Feb 10;139(6):894-906. doi: 10.1182/blood.2021012806. Blood. 2022. PMID: 34582559 Free PMC article.
-
Complexity of Blocking Bivalent Protein-Protein Interactions: Development of a Highly Potent Inhibitor of the Menin-Mixed-Lineage Leukemia Interaction.J Med Chem. 2018 Jun 14;61(11):4832-4850. doi: 10.1021/acs.jmedchem.8b00071. Epub 2018 May 23. J Med Chem. 2018. PMID: 29738674 Free PMC article.
-
Distinct pathways affected by menin versus MLL1/MLL2 in MLL-rearranged acute myeloid leukemia.Exp Hematol. 2019 Jan;69:37-42. doi: 10.1016/j.exphem.2018.10.001. Epub 2018 Oct 10. Exp Hematol. 2019. PMID: 30315824 Free PMC article.
-
The many facets of MLL1 regulation.Biopolymers. 2013 Feb;99(2):136-45. doi: 10.1002/bip.22126. Biopolymers. 2013. PMID: 23175388 Review.
-
Targeting protein-protein interaction between MLL1 and reciprocal proteins for leukemia therapy.Bioorg Med Chem. 2018 Jan 15;26(2):356-365. doi: 10.1016/j.bmc.2017.11.045. Epub 2017 Dec 1. Bioorg Med Chem. 2018. PMID: 29254892 Review.
Cited by
-
Menin in Cancer.Genes (Basel). 2024 Sep 21;15(9):1231. doi: 10.3390/genes15091231. Genes (Basel). 2024. PMID: 39336822 Free PMC article. Review.
-
Small Extracellular Vesicles Promote Stiffness-mediated Metastasis.Cancer Res Commun. 2024 May 9;4(5):1240-1252. doi: 10.1158/2767-9764.CRC-23-0431. Cancer Res Commun. 2024. PMID: 38630893 Free PMC article.
-
Local, Sustained, and Targeted Co-Delivery of MEK Inhibitor and Doxorubicin Inhibits Tumor Progression in E-Cadherin-Positive Breast Cancer.Pharmaceutics. 2024 Jul 25;16(8):981. doi: 10.3390/pharmaceutics16080981. Pharmaceutics. 2024. PMID: 39204325 Free PMC article.
References
-
- Yamaguchi H., Wyckoff J., Condeelis J., Cell migration in tumors. Curr. Opin. Cell Biol. 17, 559–564 (2005). - PubMed
-
- Georgouli M., Herraiz C., Crosas-Molist E., Fanshawe B., Maiques O., Perdrix A., Pandya P., Rodriguez-Hernandez I., Ilieva K. M., Cantelli G., Karagiannis P., Mele S., Lam H., Josephs D. H., Matias-Guiu X., Marti R. M., Nestle F. O., Orgaz J. L., Malanchi I., Fruhwirth G. O., Karagiannis S. N., Sanz-Moreno V., Regional activation of myosin II in cancer cells drives tumor progression via a secretory cross-talk with the immune microenvironment. Cell 176, 757–774.e23 (2019). - PMC - PubMed
-
- Singh S., Singh U. P., Grizzle W. E., Lillard J. W., CXCL12-CXCR4 interactions modulate prostate cancer cell migration, metalloproteinase expression and invasion. Lab. Invest. 84, 1666–1676 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

