Mechanosensing regulates tissue repair program in macrophages
- PMID: 38478620
- PMCID: PMC10936955
- DOI: 10.1126/sciadv.adk6906
Mechanosensing regulates tissue repair program in macrophages
Abstract
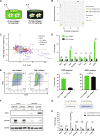
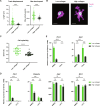
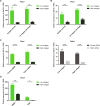
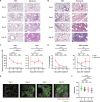
Tissue-resident macrophages play important roles in tissue homeostasis and repair. However, how macrophages monitor and maintain tissue integrity is not well understood. The extracellular matrix (ECM) is a key structural and organizational component of all tissues. Here, we find that macrophages sense the mechanical properties of the ECM to regulate a specific tissue repair program. We show that macrophage mechanosensing is mediated by cytoskeletal remodeling and can be performed in three-dimensional environments through a noncanonical, integrin-independent mechanism analogous to amoeboid migration. We find that these cytoskeletal dynamics also integrate biochemical signaling by colony-stimulating factor 1 and ultimately regulate chromatin accessibility to control the mechanosensitive gene expression program. This study identifies an "amoeboid" mode of ECM mechanosensing through which macrophages may regulate tissue repair and fibrosis.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

